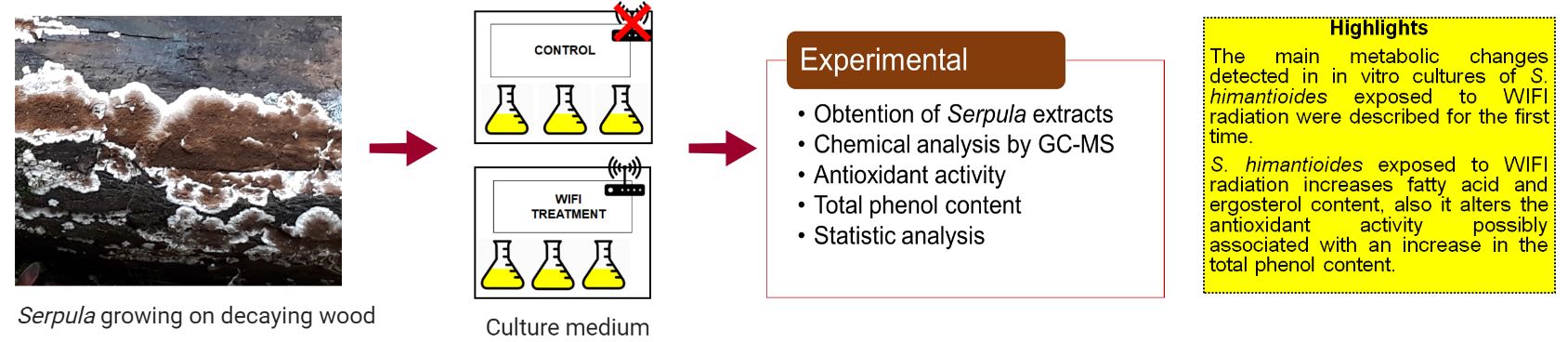
Effect of Wi-Fi radiofrequencies on the content of acid fatty and ergosterol, a precursor of vitamin D, in in vitro cultures of the fungus Serpula himantioides
- Serpula himantioides radiofrequencies Wi-Fi metabolites antioxidant activity
Copyright (c) 2021 www.jcchems.com

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
In the microwave-radiofrequency spectrum, Wi-Fi is a short-range wireless broadband technology, which transmits at a frequency of 2.5 GHz. This type of technology has been positioned itself as one of the most widely used technological advances in recent years. It has been reported that direct exposition to radiofrequencies can induce physiological changes in different animals and plants species. One of the degenerative effects Wi-Fi can cause is the production of reactive oxygen species, inducing tissue damage and DNA alterations. In fungi, the effect of exposure to electromagnetic radiation in the UV spectrum has been extensively studied, the physiological effect that exposure to radiofrequencies such as Wi-Fi might have is unknown. In that sense, the aim of this study was to detect the main changes in metabolism of some acid fatty and ergosterol of the fungus Serpula himantioides, using gas chromatography - mass spectrometry (GC–MS), and evaluate the antioxidant activity of metabolites obtained from in vitro cultures of S. himantioides exposed to Wi-Fi at 2.5 GHz. The results showed an increment in the content of some the fatty acids and ergosterol, in total extracts from the fungus mycelium. Antioxidant analysis revealed that the total extracts of S. himantioides culture medium showed antioxidant capacity associated with an increment in the content of phenolic compounds. Our results suggest that direct exposition to Wi-Fi radiation produces oxidative damage by modifying cellular components in the fungus S. himantioides.
References
- T. Obata, A.R. Fernie, Cell Mol Life Sci. 69, 3225, (2012).
- G.J. Hyland, Lancet. 356, 1833, (2000).
- J.H. Kim, J.K. Lee, H.G. Kim, K.B. Kim, K.R. Kim, Biomol. Ther. (Seoul). 27, 265, (2019).
- K.R. Foster, J.E. Moulder, Health Phys. 105, 561, (2013).
- J. Friedman, S. Kraus, Y. Hauptman, Y. Schiff, R. Seger, Biochem J. 405, 559, (2007).
- C. Avendaño, A. Mata, C.A.S Sarmiento, G.F. Doncel, Fertil Steril. 97, 39, (2012).
- K. Öngel, N. Gümral, F. Özgüner, Cell Membr. Free Radic. Res. 1, 85, (2009).
- N. Gümral, M. Naziroglu, A. Koyu, K. Ongel, O. Celik, M. Saygin, M. Kahriman, S. Caliskan, M. Kayan, O. Gencel, M. F. Flores-Arce, Biol. Trace Elem. Res.132, 153, (2009).
- M. Tkalec, K. Malarić, B. Pevalek-Kozlina, Bioelectromagnetics. 26, 185, (2005).
- D.A. Meloni, M.A. Oliva, C.A. Martinez, J. Cambraia, Environ. Exp. Bot. 49, 69, (2003).
- W. Xiujuan, W. Bochu, J. Yi, L. Defang, D. Chuanren, Y. Xiaocheng, A. Sakanishi, Colloids Surf. B Biointerfaces. 27, 59, (2003).
- H. Costa, S.M. Gallego, M.L. Tomaro, Plant Sci. 162, 939, (2002).
- M.Farooq, G.S. Babu, R.S. Ray, R.B. Misra, U. Shankar, R.K. Hans, Biochem. Biophys. Res. Commun. 276, 970, (2000).
- H.X. Ren, Z.L. Wang, X. Chen, Y.L. Zhu, Environ. Exp. Bot. 42, 51, (1999).
- Y.Y. Su, Y.L. Qi, L. Cai, Mycology. 3, 195, (2012).
- K.A. Hugues, B. Lawley, K.K. Newsham, Appl. Environ. Microbiol. 69, 1488, (2003).
- A. Fourtouni, Y. Manetas, C. Christias, Can. J. Bot. 76, 2093, (1998).
- S. Torres, M. González-Ramírez, J. Gavilán, C. Paz, G. Palfner, N. Arnold, J. Fuentealba, J. Becerra, C. Pérez, J.R.Cabrera-Pardo. Appl. Environ. Microbiol. 85, e00870-19, (2019).
- T. Carlsen, I.B. Engh, C. Decock, M. Rajchenberg, H. Kauserud, Fungal biol. 115, 54, (2011).
- P. Aqueveque, T. Anke, O. Sterner, Z. Naturforsch C. J. Biosci. 57, 257, (2002).
- M. Gill, W. Steglich Pigments of fungi (Macromycetes). In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products, Springer, Vienna, 1987.
- Y. Chen, Z. Cai, Q. Feng, P. Gao, Y. Yang, X. Bai, B.Q. Tang, Cogent Biol. 5, 1, (2019).
- A. Shahin-jafari, M. Bayat, M.H. Shahhosseiny, P. Tajik, S. Roudbar-mohammadi, Saudi J Biol Sci. 23, 426, (2016).
- S. Shokri, A. Soltani, M. Kazemi, D. Sardari, F.B. Mofrad, Cell J. 17, 322, (2015).
- M.L. Soran, M. Stan, Ü. Niinemets, L. Copolovici, J. Plant Physiol. 171, 1436, (2014).
- K.T. Lim, C. Hu, D.D. Kitts, Food Chem. Toxicology. 39, 229, (2001).
- N. Nenadis, L.F. Wang, M. Tsimidou, H.Y. Zhang, J. Agric. Food Chem. 52, 4669, (2004).
- V.L. Singleton, J.A. Rossi, Am. J. Enol. Vitic. 16, 144, (1965).
- K. Knight, J. Exp. Biol. 217, 1, (2014).
- M. Suutari, K. Liukkonen, S. Laakso, J Gen Microbiol. 136, 1469, (1990).
- A. Vian, E. Davies, M. Gendraud, P. Bonnet, Biomed Res. Int. 2016, 1, (2016).
- S. Banik, S. Bandyopadhyay, S. Ganguly, Bioresour. Technol. 87, 155, (2003).
- M.H. Gordon FATS|Classification. In Encyclopedia of Food Sciences and Nutrition, Oxford: Academic Press, 2003.
- U. Krings, R.G. Berger, Food Chem. 149, 10, (2014).
- A. Teichmann, P.C. Dutta, A. Staffas, M. Jägerstad, LWT Food Sci.Technol. 40, 815, (2007).
- V.J. Jasinghe, C.O. Perera, Food chem. 92, 541, (2005).
- S. Shao, M. Hernandez, J.K. Kramer, D.L. Rinker, R. Tsao, J. Agric. Food Chem. 58, 11616, (2010).
- J.A. Ko, B.H. Lee, J.S. Lee, H.J. Park, 2008. J. Agric. Food Chem. 56, 3671, (2008).
- M.S. Calvo, U.S. Babu, L.H. Garthoff, T.O Woods, M. Dreher, G. Hill, S. Nagaraja, Osteoporos Int. 24, 197, (2013).
- R.R. Simon, J.F. Borzelleca, H.F. De Luca, C.M. Weaver, Food Chem. Toxicol. 56, 278, (2013).
- H.P. Singh, V.P. Sharma, D.R. Batish, R.K. Kohli, Environ. Monit. Assess. 184, 1813, (2012).
- S.J. Huang, C.P. Lin, J.L. Mau, Y.S. Li, S.Y. Tsai, Int. J. Med. Mushrooms. 17, 241, (2015).
- Â. Fernandes, A.L. Antonio, M.B. Oliveira, A. Martins, I.C. Ferreira, Food Chem. 135, 641, (2012).
- K. Akram, J.H. Kwon, J. Korean Soc. Appl. Biol. Chem. 53, 257, (2010).
- T. Jiang, M.M. Jahangir, Z. Jiang, X. Lu, T. Ying, Postharvest Biol. Technol. 56, 209, (2010).
- I. Sommer, H. Schwartz, S. Solar, G. Sontag, J. Agric. Food Chem. 57, 5790, (2009).
- W. Guan, X. Fan, R. Yan, Postharvest Biol. Technol. 64, 119, (2012).