SYNTHESIS AND ANTIMICROBIAL STUDY OF NEW BENZIMIDAZOLE SCHIFF BASES BEARING P-TOLUENE SULFONAMIDE MOIETY

- Benzimidazole,
- Schiff bases,
- antimicrobial activity.
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
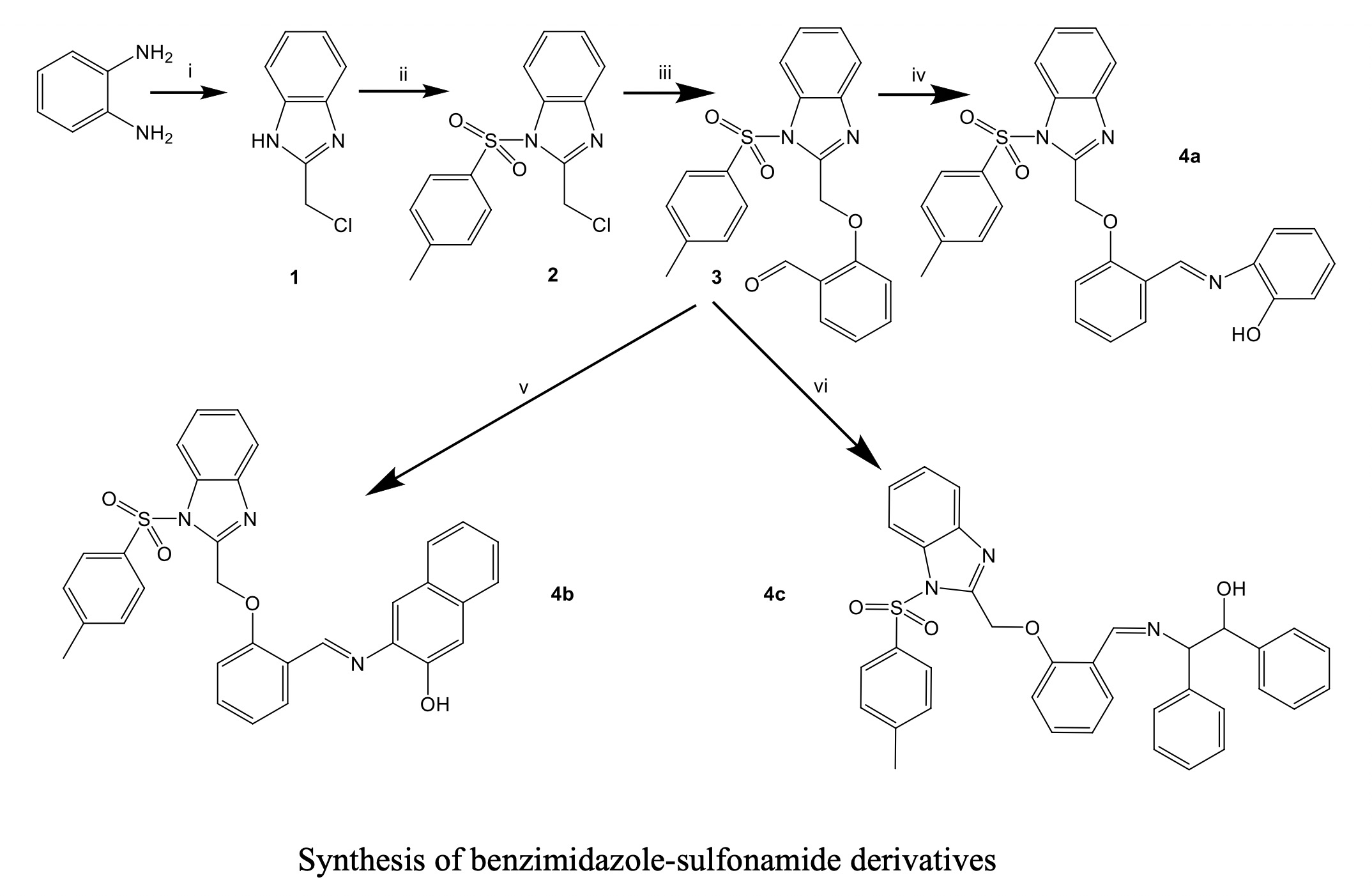
Three new Schiff bases bearing benzimidazole and p-toluen sulfonamide moiety were synthesized. Their structures were characterized by elemental analysis, FTIR, NMR and LCMS methods. The antimicrobial properties of the compounds were also evaluated. All compounds exhibit higher antimicrobial activity against all bacteria, Schiff bases (4a-4c) shows more activity than the parent aldehyde (3). 4c displays higher antibacterial activity against P. aeruginosa compare to reference drug Sulfioxazole. The compounds were found more potent against Gram-negative than Gram positive bacteria. One more free phenyl group increase the activity in 4c, but one more phenyl ring in 4b does not.In vitro antifungal activity of the compounds was also tested on fungi C. albicans C. tropicalis and C. krusei. However, our compounds exhibit no antifungal activity against three fungi.
References
- . Z.A. Alagoz, S. Yıldız, E, Buyukbingol, Chemotherapy, 53, 110, (2007)
- . S.O. Podunavac-Kuzmanovic, D.M. Cvetkovic, J. Serb. Chem. Soc. 75, 459, (2007)
- . R.S. Keri, C.K. Rajappa, S.A. Patil, B.M. Nagaraja, Pharmacol. Rep, 68, 1254 (2016)
- . S.T. Asundaria, K.C. Patel, Pharm. Chem. J. 45, 725, (2012)
- . B. Soni, M.S. Ranawat, R. Sharma, A. Bhandari, S. Sharma, Eur. J. Med. Chem. 45, 2938, (2010)
- . A. Gellis, H. Kovacic, N. Boufatah, P. Vanelle, Eur. J. Med. Chem. 43, 1858, (2008)
- . H. M. Refaat, Eur. J. Med. Chem. 45, 2949, (2010)
- . K. Starcevic, M. Kralj, K. Ester, I. Sabol, M. Grce, K. Pavelic, G. Karminski-Zamola, Bioorg. Med. Chem. 15, 4419, (2007)
- . S. Saeed, N. Rashid, P. G. Jones, M. Ali, R. Hussain, Eur. J. Med. Chem. 45, 1323, (2010)
- . D. Havrylyuk, L. Mosula, B. Zimenkovsky, O. Vasylenko, A. Gzella, R. Lesyk, Eur. J. Med. Chem. 45, 5012 (2010)
- . R. Caputo, M.L. Calabro, N. Micale, A.D. Schimmer, M. Ali, M. Zappala, S. Grasso, Med. Chem. Res. 21, 2644 (2012)
- . D. Evans, T.A. Hicks, W.R.N. Williamson, W. Dawson, S.C.R. Meacock, E.A. Kitchen, Eur. J. med. Chem. 31, 635 (1996)
- . M. Gaba, D. Singh, S. Singh, V. Sharma, P. Gaba, Eur. J. med. Chem. 45, 2245 (2010)
- . S. Bhattacharya, P. Chaudhuri, Current Medicinal Chemistry, 15, 1762 (2008)
- M.E. Wolff, (Ed.), Burger’s Medicinal Chemistry and Drug Discovery, 4rd ed.; John Wiley & Sons: Laguna Beach, 1996
- J. Lal, S.K. Gupta, D. Thavaselvam, D.D. Agarwal, Eur. J. Med. Chem. 64, 579 (2013)
- R. Gawin, E. De Clercq, L. Naesens, M. Koszytkowska-Stawinska, Bioorg. Med. Chem. 16, 8379 (2008)
- V. Akurathi, L. Dubois, S. Celen, N.G. Lieuwes, S.K. Chitneni, B.J. Cleynhens, A. Innocenti, C.T. Supuran, A.M. Verbruggen, P. Lambin, G.M. Bormans, Eur. J. Med. Chem. 71, 374 (2014)
- N. Chandna, S. Kumar, P. Kaushik, D. Kaushik, S.K. Roy, G.K. Gupta, S.M. Jachak, J.K. Kapoor, P.K. Sharma, Bioorg. Med. Chem. 21, 4581 (2013)
- W.O. Foye, T.L. Lemke, D.A. Williams, Principles of Medicinal Chemistry, 4rd ed., Williams & Williams, 1995.
- X.J. Fang, P. Jeyakkumary, S.R. Avula, Q. Zhou, C.H. Zhou, Bioorg. Med. Chem. Lett. 26, 2584 (2016)
- H.Z. Zhang, S.F. Cui, S. Nagarajan, S. Rasheed, G.X. Cai, C.H. Zhou, Tetrahedron Lett. 55, 4105 (2014)
- J. Ponmani, H.B. Liu, L. Gopala, Y. Cheng, X.M. Peng, R.X. Geng, C.H. Zhou, Bioorg. Med. Chem. Lett. 27, 1737 (2017)
- . N. Siddiqui, Md.S. Alam, R. Ali, M. Shahar Yar, O. Alam. Med. Chem. Res., 25, 1390 (2016)
- . K.B. Abdireimov, N.S. Mukhamedov, R. Ya Okmanov, M.Zh. Aiymbetov, B. Tashkhodzhaev, Kh.M. Shakhidoyatov, Chem. of Heterocyclic Compounds, 49, 760 (2013)

