SYNTHESIS AND X-RAY STRUCTURAL CHARACTERIZATION OF 1-(5-BROMOBENZOFURAN-2-YL)-2- MESITYLETHANONEOXIME AND 1-(5-BROMOBENZOFURAN-2-YL)-2-MESITYLETHANONE-O- (2- PHENYLACETYL) OXIME
- Benzofuran derivatives,
- Oxime derivatives,
- Crystal structure,
- X-ray,
- 2D Network
Copyright (c) 2017 Gül Yakali, Esra Barim, Cumhur Kirilmiş, Muhittin Aygün

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
The selected molecules of benzofuran derivatives were synthesized and their structures were studied using the X-ray crystallography and spectroscopic methods. The 1-(5-bromobenzofurane-2-yl)-2-mesitylethanoneoxime (I), C19H18BrNO2, and 1-(5-bromobenzofuran-2-yl)-2-mesitylethanone-O-(2-phenylacetyl) oxime (II), C27H24BrNO3, crystallize in the monoclinic crystal system in space group P21/c with Z = 4 and in the triclinic system in space group P-1 with Z=2, respectively. The both compounds adopt Z conformation. The compound (I) consists of a dimeric arrangement of molecules around an inversion centre formed via a O-H…N intermolecular hydrogen bond linking the molecules – Along the a axis. This centrosymmetric hydrogen-bonded dimers are formed with an R 2/2 (6) ring motif. The compound (II) forms one dimensional infinite chain via C–H···O hydrogen bond along the a axis. Moreover, in the crystal structures of (I), (II) weak C-H..O, C-H…π and π…π interactions serve to organize formation of a two dimensional network. Moreover a short N1-N1 intermolecular contact [2.89 Å] is observed between inversion-related chains in the crystal structure of (I). The two compounds have essentialy similar bond lengths and angles. The prominent discrepancy is observed for the fragment attaching benzofuran ring with mesityl group. Benzofuran groups and other ring groups are almost planar in the crystal structures of (I) and (II).
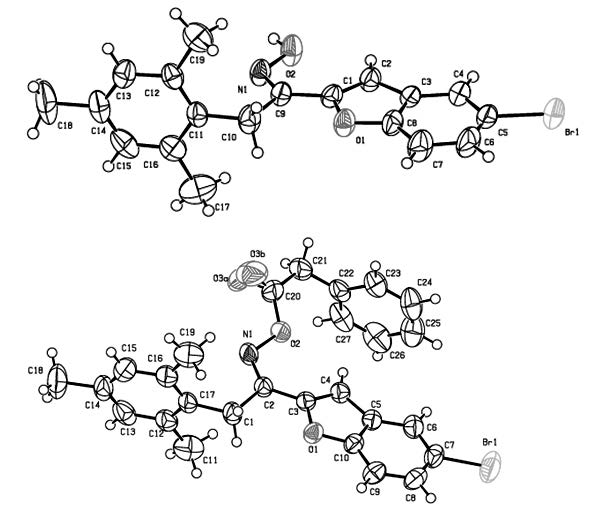
References
- H. Khanam, Shamsuzzaman, 2015, European Journal of Medicinal Chemistry, 97, 483-504.
- T.L. Gilchrist, 1985, Heterocyclic Chemistry, 174.
- J. Kalia, R.T. Raines, 2008, Angew. Chem. Int. Ed., 47, 7523–7526.
- M. Kamal, K. A. Shakya, T. Jawaıd, International Journal of Medicaland Pharmaceutical Sciences, 2011, 1-3.
- C. Kirilmis, M. Ahmedzade, S. Servi, M. Koca, A. Kizirgil, C. Kazaz, European Journal of Medicinal Chemistry, 2008, 43, 300-308.
- V. N. Telvekar, H. S. Kundaikar, K. N. Patel, H. K. Chaudhari, QSAR Comb. Sci., 2008, 27, 1193-1203.
- N. G. Karaburun, K. Benkli, Y. Tunali, Ü. Uçucu, Ş. Demirayak, European Journal of Medicinal Chemistry, 2006, 1, 651–656.
- K. Mehnaz, A. K. Shakya, J. Talha , International Jounal of Medical and Pharmaceutical Sciences, 2011, 13, 1-15.
- V. Bertalosi, G. Gilli, A. Veronese, Acta. Cryst. B, 1982, 38, 502−511.
- E. A. Bruton, L. Brammer, F. C. Pigge, C. B. Aakeröy, D. S. Leinen, New J. Chem., 2003, 27, 1084−1094.
- J. N. Low, L. M. N. B. F. Santos, C. F. R. A. C. Lima, P. Brandão, L. R. Gomes, Eur. J. Chem., 2010, 1, 61−66.
- C. B. Aakeröy, A. S. Sinha, K. N. Epa, P. D. Chopade, M. M. Smith, J. Desper, Crystal Growth Design, 2013, 13, 2687-2695.
- O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H. Puschmann, J. Appl. Cryst., 2009, 42, 339-341.
- G.M. Sheldrick, Acta Cryst. A, 2008, 64, 112.
- M.N. Burnett, C.K. Johnson, ORTEPIII. Report ORNL-6895, Oak Ridge National Laboratory, TN, USA, 1996.
- J. Bernstein, R. E Davis, L. Shimoni, N. L. Chang, 1995, Angew. Chem. Int. Ed. Engl., 34, 1555–1573.
- CrysAlis PRO and CrysAlis RED. Agilent Technologies, Yarnton, Oxfordshire, England, 2002.
- V. T. Yilmaz, C. Kazak, C. Kirilmis, M. Kocac and F.W. Heinemannd, Acta Cryst.C, 2005, 61, o438-o441.
- R. Koner, I. Goldberg, 2008, Acta Cryst. C, 65,m37-m41.
- H.D. Choi, P. J. Seo, U. Lee, 2013, Acta Cryst. E, 69, o1135.
- H.D. Choi, P.J. Seo, B.W. Sonb, U. Leeb, 2006, Acta Cryst. E, 62, o2335– o2336.
- C. Arıcı, D. Ülkü, M. Koca, M. Ahmedzade, 2004, Acta Cryst. E, 60, o94-o942.
- L. Chen, Q.B. Xu, G.L.Song, H.J. Zhu, 2009, Acta Cryst. E 65, o1083.
- G. Anuradha, G. Vasuki, I. A. Khan, M.V. Kulkarni, 2012, Crystal Structure Theory and Applications, 1, 107-113.
- J. Sepencer, A.P. Mendham, B.Z. Chowdhry, R.A. Palmer, D.R.Lisgarten, B.M. Trost, N. Cramer, L. Male, S.J. Coles, 2014, Journal of Pharmaceutical Chemistry, 1(3), 44-49.
- A. Drzewiecka, A.E. Koziol, T.P. Ruiz, M. F. Gomez, M. Struga, J. Kossakowski, K. Ostrowska, T. Lis, 2012, Original Research, 23, 1617– 1629.
- R. Gowda, V.B. Jadhav, M. V. Kulkarni, K. V. A. Gowda, 2009, X-ray Structure Analysis Online, VOL. 25.
- J. Kossakowski, M. Krawiecka, B. Kuran, J. Stefańska, I. Wolska, 2010, Molecules, 15, 4737-4749.
- H. M. Titi, I. Goldberg, 2010, CrystEngComm, 12, 3914–3922.
- M. Krawiecka, B. Kuran, J. Kossakowski, I. Wolska, M. Kierzkowska, G. Meynarczyk, 2012, Polish Pharmaceutical Society, Vol. 69 No.6 1055- 1065.
- W. W. Hanand, J.L. Wu, 2014, Z. Kristallogr. NCS, 229, 27-28.
- Y. Ooyama, T. Mamura, K. Yoshida, 2006, Chem. Eur. J., 12, 7827–7838.


