- New phenolic structure,
- Benzoic acid derivative,
- Flavonoids,
- Chilean plants,
- Heliotropium
- NMR,
- X-ray diffraction ...More
Copyright (c) 2017 Iván Brito, Mario J. Simirgiotis, Maria Avendaño, Alejandra Bravo, Jorge Borquez, Alejandro Cárdenas

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
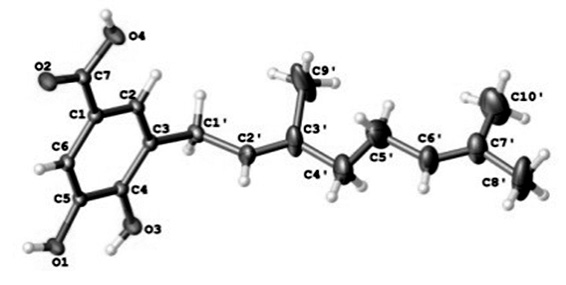
The crystal structure of the aromatic compound 3,4-dihydroxy-5-geranyl-benzoic acid (5) is reported for the first time. This compound was isolated from the resinous exudate of Manto Negro (Heliotropium taltalense I. M. Johnst), an endemic species growing in The Atacama Desert using a combination of High speed counter current chromatography (HSCCC) and permeation through Sephadex LH-20. The structure was elucidated by spectroscopic means and confirmed by X-ray crystal-structure analysis. All OH groups are involved in hydrogen bonding with the average H⋅⋅⋅O distance of 2.760(3) Å and O-H⋅⋅⋅O angles in the range 125-159°, so in the crystal packing the molecules are associated by three strong intermolecular hydrogen bonds forming ring with graph-set motif R2²(14) R2²(6).
References
- B. Modak, M. Rojas, R Torres, Molecules 1980, 14, (2009).
- R. Torres, L Villaroel, A Urzua, F Delle Monache, G Delle Monache, E. Gacs-Baitz. Phytochemistry, 249, 36, (1994).
- K. Skalicka-Wozniak, I. Garrard, Phytochem Ver. 547, 13, (2014).
- M. J. Simirgiotis, J. Bórquez, M. Neves-Vieira, I. Brito, S. Alfaro-Lira, P. Winterhalter, C. Echiburú-Chau, G. Jerz, A. Cárdenas, Ind. Crops Prod. 76, 69, (2015).
- J. Bórquez, E. J. Kennelly, M. J. Simirgiotis, Food Res. Int. 52, 288, (2014).
- P. K. Agrawal, Carbon-13 NMR of flavonoids. Michigan, Elsevier. 1989.
- M. Gajhede, E. R. Leal, G. C. Patino, J. C. Christophersen, P. H. Nielsen, Acta Cryst. 2012, C45, (1989).
- R. Torres, B. Modak, L. Villarroel, A. Urzua, F. Delle-Monache, Bol. Soc. Chil. Quím. 195, 41, (1996).
- H. D. Smolarz, E. Mendyk, A. Bogucka-Kocka, Zeitsch. Naturforsch., 64, 61c, (2006).
- R. D. Torrenegra, O. E. Rodriguez, Nat. Prod. Commun. 947, 6, (2011).
- G. M. Sheldrick, Acta Cryst. 112 , A64, (2008).
- O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H. Pus¬chmann, J. Appl. Cryst.339, 42, (2009).
- N. Flores, I. A. Jiménez, A. Giménez, G. Ruiz, D. Gutiérrez, G. Bourdy, I. L. Bazzocchi, Phytochemistry 621, 70, (2009).
- T. P. Green, E. M. Treadwell, D. F. Wiemer. J. Nat. Prod. 367, 62, (1999).
- N. Flores, I. A. Jiménez, A. Giménez, G. Ruiz, D. Gutiérrez, G. Bourdy, I. L. Bazzocchi, J. Nat. Prod. 1538, 71, (2008).


