SOLID PHASE EXTRACTION AND LC-MS/MS METHOD FOR QUANTIFICATION OF VENLAFAXINE AND ITS ACTIVE METABOLITE O-DESMETHYL VENLAFAXINE IN RAT PLASMA
- Venlafaxine,
- O-desmethyl venlafaxine,
- Pharmacokinetics,
- Solid phase extraction,
- LC-MS/MS
Copyright (c) 2017 Sarfaraz Ahmad, Masoom R. Siddiqui, MD. S. Ali, Saikh M. Wabaidur, MD. S. Alam, Nawazish Alam, Zeid A. Alothman, Moonis Ali Khan, Mohammad R. Khan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
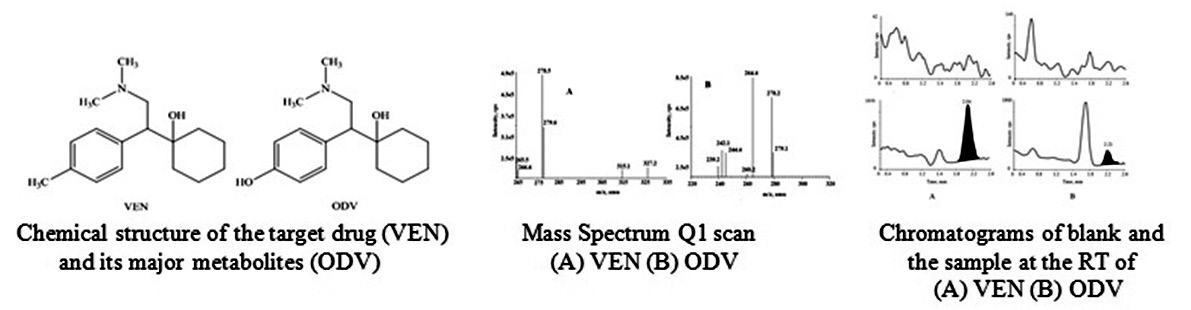
A rapid, simple, sensitive LC-MS/MS method involving a least pretreatment process has been proposed for the quantitative assessment of venlafaxine (VEN) and O-desmethyl venlafaxine (ODV) using cetirizine as an internal standard. The method was validated over the range of 1.03 ng/mL to 453.50 ng/mL (venlafaxine) and 1.32 ng/mL to 585.21 ng/mL (O-desmethyl venlafaxine). The lowest limit of quantification for venlafaxine and O-desmethyl venlafaxine was found to be 1.03 ng/mL and 1.32 ng/mL, respectively. The solid phase extraction procedure provided reliable and reproducible recoveries of the drug as well as its active metabolite with no interference at their retention time. The recovery for all analyzed drugs was found to be in the range of 72.55% to 74.75%. The result indicates that the developed procedure could be considered suitable for carry out simultaneous preclinical pharmacokinetics studies for VEN and ODV.
References
- I. M. Whyte, A. H. Dawson and N. A. Buckley, QJM-Int J Med. 96, 369, (2003).
- E.A. Muth, J.T. Haskins, J. A. Moyer, G. E. Husbands, S. T. Nielsen and E. B. Sigg, Biochem. Pharmacol. 35, 4493, (1986).
- F. P. Bymaster, L. J. Dreshfield-Ahmad, P. G. Threlkeld, J. L. Shaw, L. Thompson, D. L. Nelson , S. K. Hemrick-Luecke and D. T. Wong, Neuropsychopharmacology, 25, 871, (2001).
- S. R. Howell, G. E. M. Husbands, J. A. Scatina and S. F. Sisenwine, Xenobiotica, 23, 349, (1993).
- Mayo Clinic staff (2005). “Beyond hormone therapy: Other medicines may help”. Hot flashes: Ease the discomfort of menopause. Mayo Clinic. Retrieved 20 january 2015.
- C. E. Schober and N. T. Ansani, Ann Pharmacother. 37, 1703, (2003).
- D. R. Grothe, B. Scheckner and D. Albano, Pharmacotherapy, 24, 621, (2004).
- E. A. Muth , J. A. Moyer, T. Haskins, T. H. Andree and G. E. M. Husbands, Drug Dev. Res. 23,191, (1991).
- S. Cherkaoui, S. Rudaz, E. Varesio and J.-L. Veuthey, Electrophoresis, 22, 491, (2001).
- S. Cherkaoui, S. Rudaz, E. Varesio and J.-L. Veuthey, Electrophoresis, 22, 3308, (2001).
- S. Rudaz, C. Stella, A. E. Balant-Gorgia, S. Fanali and J. L. Veuthey, J. Pharm. Biomed. Anal. 23, 107, (2000).
- S. Rudaz, J. L. Veuthey, C. Desiderio and S. Fanali, Chromatographia, 50, 369, (1999).
- L. Labat, M. Deveaux, P. Dallet and J. P. Dubost. J. Chromatogr. B., 773,17, (2002).
- G. R. Shah, B. T. Thaker, K. R. Surati and M. H. Parabia, Anal. Sci. 25, 1207, (2009).
- J. Bhatt, A. Jangid, G. Venkatesh, G. Subbaiah and S. Singh. J. Chromaotr. B. 829, 75, (2005).
- S. K. Dubey, R. N. Saha, H. Jangala and S. Pasha, J. Pharm. Anal. 3, 466, (2013).
- K. F. Ilett, J. H. Kristensen, L. P. Hackett, M. Paech, R. Kohan and J. Rampono, Braz. J. Clin. Pharmacol. 53, 17, (2002).
- M. A. Matoga, F. B. Pehourcq, K. B. Titier, F. B. Dumora, and C. A. Jarry, J. Chromatogr. B. 760, 213, (2001).
- L. Dutta, S. I. Ahmad, S.K. Mukherjee, S. Mishra, A. Khuroo, and T. Monif, Biomed. Chromatogr. 27, 622, (2013).
- D. C. Reddy , A. T. Bapuji, V.S. Rao, V. Himabindu and S. Ravinder, J. Liq. Chromatogr. & Rel. Technol. 36, 897, (2013).
- L. Kang, H. W. Jun, and J. W. McCall, J. Pharm. Biomed. Anal. 19, 737, (1999).
- X. Wang, J. A. Paul, T. N. Nanovskaya, G. D. V. Hankins and M. S. Ahmed, J. Pharm. Biomed. Anal. 98, 107, (2014).


