EVALUATION OF MONOLITHIC COLUMN FOR INORGANIC MERCURY AND METHYLMERCURY DETERMINATION IN FISH SAMPLE ANALYSIS
- Mercury speciation,
- Monolithic column,
- Fish samples
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
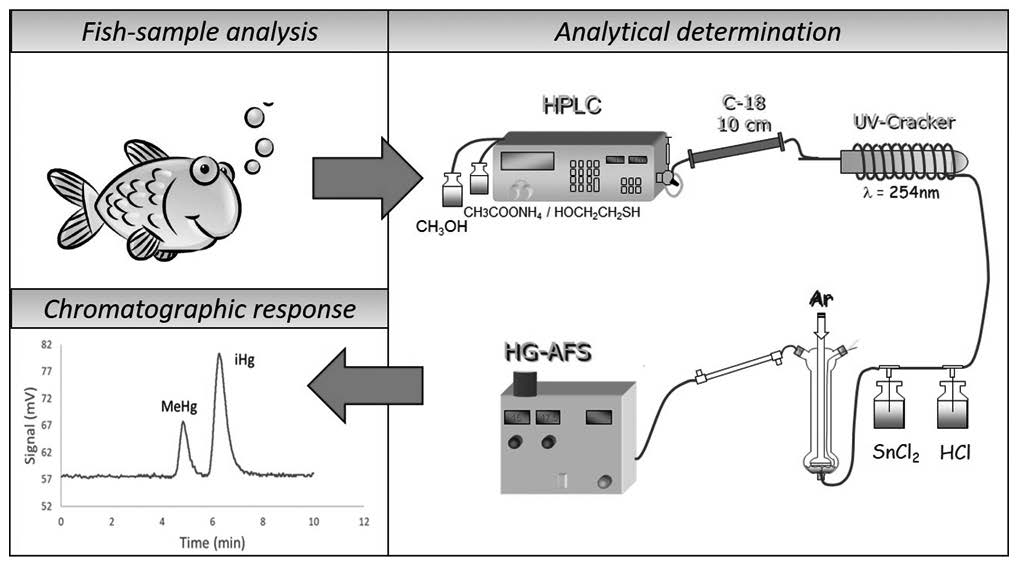
In the present work, the applicability of a monolithic column was evaluated for mercury speciation analysis in certified and real fish samples. The influence of the flow and composition of the mobile phase (2-mercaptoethanol concentration and methanol content) on the retention times of iHg and MeHg was evaluated. The optimal separation conditions were achieved for a mobile phase containing 5 % methanol and 0.02 % mercaptoethanol in acetate buffer (pH 5.0) at a flow rate of 1.6 ml min-1. Different extraction conditions were studied to analyze a certified reference material with the optimized methodology and the best results (93 % recovery) were obtained for microwave-assisted extraction with a mixture of HCl 6 M + NaCl 0.5 M. Finally, the proposed method was satisfactorily applied to real fish samples collected from different markets in the central region of Chile, presenting levels ranging from 0.73 – 3.4 mg kg-1.
References
- (1) Sakamoto, K. M.; Kakita, A.; Masanori, S.; Environ Toxicol Chem. 2012, 501-516.
- (2) Dopp, E.; Hartmann, M.; Flores, M.; Rettenmeier, W.; Hirner, A. V.; Crit Rev Toxicol. 2004, 34, 301-333.
- (3) Bjornberg, K. A.; Vahtera, M.; Grawe, K. P.; Berglund, M.; Sci Total Environ. 2005, 341, 45-52.
- (4) Lindberg, A.; Bjornberg, K. A.; Vahter, M.; Berglund, M.; Environ Res.2004, 96, 28-33.
- (5) Björkman, L.; Lundekvam, B. F.; Lægreid, T.; Bertelsen, B. I.; Morild, I.; Lilleng, P.; Lind, B.; Palm, B.; Vahter, M.; Environ Health 2007.
- (6) Chen, Y. W.; Belzile, N.; Anal Chim Acta 2010, 671, 9-26.
- (7) Clemens, S.; Monperrus, M.; Donard, O. F. X.; Amouroux, D.; Guerin, T.; Talanta 2012, 89, 12-20.
- (8) Chen, X. P.; Han, C.; Cheng, H. Y.; Liu, J. H.; Xu, Z. G.; Yin, X. F.; Anal Chim Acta 2013, 796, 7-13.
- (9) Hirner, A. V. Anal Bioanal Chem.; 2006, 385, 555-567.
- (10) Carrasco, L.; Vassileva, E.; Talanta 2014, 122, 106-114.
- (11) Clevenger, W. L.; Smith, B. W.; Winefordner, J. D.; Crit Rev Anal Chem 1997, 27, 1-26.
- (12) Sanchez-Rodas, D.; Corns, W. T.; Chen, B.; Stockwell, P. B.; J. Anal Atomic Spectrometry 2010, 25, 933-946.
- (13) Xu, X. M.; Liu, R.; Wei, C.; Qiu, W.; Gao, Y.; Atomic Spectroscopy 2013, 34, 238-243.
- (14) Jin, L.; Liu, J. F.; Ye, B. X.; Ren, A. G.; Environ Inter 2014, 66, 157-164.
- (15) Houserova, P.; Matejicek, D.; Kuban, V.; Pavlickova, J.; Komarek. J.; J. Separation Sci 2006, 29, 248-255.
- (16) Jia, X. Y.; Han, Y.; Liu, X. L.; Duan, T. C.; Chen, H. T.; Spectrochim Acta Part B At Spectrosc 2011, 66, 88-92.
- (17) de Souza, S. S.; Rodriguez, J. L.; Souza, V. C. D.; Barbosa, F.; J. Anal Atomic Spectrometry 2010, 25, 79-83.
- (18) Namera, A.; Saito, T.; Trends Analyt Chem 2013, 45, 182-196.
- (19) Cabrera, K.; Trends Analyt Chem 2004, 27, 843-852.
- (20) Aggarwal, P.; Tolley, H. D.; Lee, M. L.; J. Chromatog A 2012, 1219, 1-14.
- (21) Siouffi, A. M.; J. Chromatog A 2003, 1000, 801-818.
- (22) Scancar, J.; Milacic, R.; Trends Anal. Chem 2009, 28, 1048-1056.
- (23) Milacic, R.; Zuliani, T.; Vidmar, J.; Scancar, J.; J Anal Atom Spectrom 2016, 31, 1766-1779.
- (24) Thompson, M.; Ellison, S. L. R.; Wood, R.; J. Pure Appl. Chem 2002, 74, 835-855.


