- Azorella compacta,
- Apiaceae,
- diterpenoids,
- antibacterial activity
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
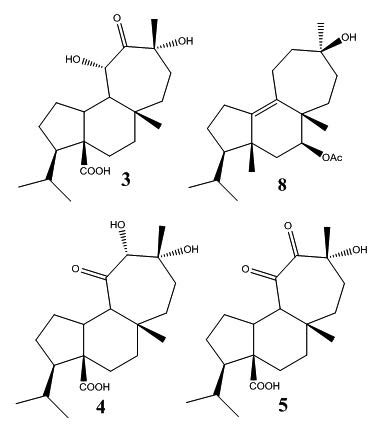
The antibacterial activity of two natural diterpenoids isolated from Azorella compacta together with six semisynthetic derivatives, were evaluated against three bacteria: Staphylococcus aureus, Escherichia coli and Mycobacterium smegmatis. The synthesis of the semisynthetic derivatives 3-5 and 8 have not been previously reported with the methods described in this research. The structures of all diterpenoids were elucidated by NMR 1H, 13C and IR spectroscopy. The natural diterpenoids, mulinolic ácid (1) and azorellanol (2) did not present antibacterial activity, but the six derivatives: 12-oxo-11,13-α,α-dihydroxymulin-20-oic ácid (3), 11-oxo-12,13-α,α-dihydroxymulin-20-oic ácid (4) 11,12-dioxo-13-α-hydroxymulin-20-oic ácid (5), 7-acetoxymulin-9,12-diene (6), mulin-9,12-dien-7-ol (7) and 7-acetoxy-12,13-dihydroxymulin-9-en (8) were active against three tested bacteria. The antibacterial activity reported for six semisynthetic diterpenoids may not be comparable with positive control ampicillin but demonstrate the possibility of modification of the biological activity of diterpenoids.
References
- Castro V, Villagrán C. Ciencia indígena de los Andes del norte de Chile, Universitaria, Santiago, 2004.
- Melcher E. El norte de chile: su gente, desiertos y volcanes, Universitaria, Santiago, 2004.
- Belov M. Azorella compacta. [accessed 25 May 2016].
- http://www.chileflora.com/Florachilena/FloraSpanish/HighResPages/ SH2013.htm (2013).
- C. Kleier, P. Rundel, Austral Ecol 29, 461, (2004).
- Y. Gebhardt, G. Forkmann, R. Lukacin, S. Martens, U. Matern, S. Witte, Phytochemistry 66, 1273, (2005).
- K. Brandt, L. Christensen, J. Pharm. Biomed. Anal. 41, 683, (2006).
- M. Budêsínsky, M. Holub, Phytochemistry 25, 2015, (1986).
- J. Cherng, W. Chiang, L. Chiang, Food Chem. 106, 944, (2008).
- S. Razavi, G. Zarrini, Russ. J. Bioor. Chem. 36, 359, (2010).
- E. Ellmerer, M. Ganzera, R. Greil, K. Jöhrer, J. Mader, B. Schubert, E. Sigmund, H. Stuppner, C. Zidorn, J. Agric. Food Chem. 53, 2518, (2005).
- J. Harrorne, C. Williams, Phytochemistry 11, 1741, (1972).
- F. Abolhasani, B. Nickavar, Pak. J. Pharm. Sci. 22, 30, (2009).
- G. Arias, E. Stashenko, R. Torres, Scientia Et Technica año XIII, 75, (2007).
- A. Netrusov, I. Parshikov, J. Sutherland, Biotechnol. Adv. 30, 1516, (2012).
- A. Ardiles, G. Molina-Salinas, J. Bórquez, I. González-Collado, L. Loyola, S. Said-Fernández, A. San-Martín, L. Peña-Rodríguez, Fitoterapia 81, 50, (2010).
- A. Ardiles, P. Becerril-Montes, J. Bórquez, F. Escalante-Erosa, I. González- Collado, L. Loyola, G. Molina-Salinas, S. Said-Fernández, A. San Martín, L. Peña-Rodríguez, A.Yam-Puc, Phytochem. Rev. 9, 271, (2010).
- I. Neira, L. Poblete, P. Porcille, P. Silva, J. Araya, J. Bórquez, G. Morales, L. Loyola, H. Sagua, Bol. Chil. Parasitol. 53, 9, (1998).
- J. Bórquez, N. Fuentes, L. Loyola, G. Morales, H. Sagua, A. San Martín, J. Soto, Phytother. Res. 19, 713, (2005).
- N. Backhouse, J. Bórquez, C. Delporte, L. Loyola, P. Salinas, A. San- Martín, Bioorg. Med. Chem. Lett. 11, 1187, (2003).
- P. Becerril-Montes, J. Bórquez, F. Escalante-Erosa, L. Loyola, G. Molina- Salinas, L. Peña-Rodríguez, S. Said-Fernández, A. Yam-Puc, Fitoterapia 81, 219, (2010).
- J. Benites, P. Díaz, J. López, J. Ordóñez, E. Pastene, M. Rojas, L. Rojo, B. Latinoam. Caribe Pl. 8, 498, (2009).
- C. Areche, C. Campos-Briones, C. Lima, E. Pérez, F. Rojas-Alvarez, B. Sepúlveda, J. Pharm. Pharmacol. 65, 1231, (2013).
- J. Bórquez, L. Loyola, V. Manriquez, G. Morales, A. San-Martin, O. Wittke, Tetrahedron 54, 15533, (1998).
- A. Loyola, J. Bórquez, G. Morales, A. San-Martin, Phytochemistry 43, 165, (1996).


