Mn2+-COMPLEXES OF N,O-DIHYDRAZONE: STRUCTURAL STUDIES, INDIRECT BAND GAP ENERGY AND CORROSION INHIBITION ON ALUMINUM IN ACIDIC MEDIUM
- Metal complexes,
- Hydrazones,
- Structure elucidation,
- Corrosion
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
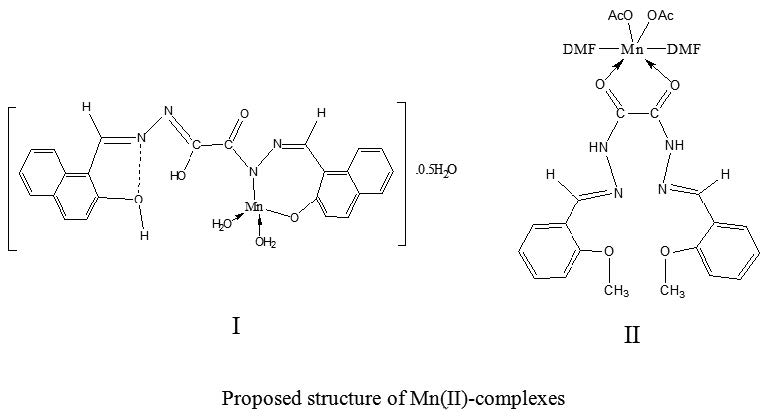
Mononuclear manganese complexes of dihydrazone derived from the condensation of oxaloyldihydrazide with 2-hydroxynaphthaldehyde and 2-methoxybenzaldehyde have been synthesized. The hydrazone Schiff base ligands and their chelates were characterized on the basis of their elemental analyses, spectral (UV-Vis., IR, mass, 1H/13C NMR), magnetism, ESR and thermal (TGA) measurements. The dihydrazone has been suggested to coordinate to the metal center in bi-dentate manner forming 1:1[M:L] complex. The complexes are suggested to have tetrahedral/octahedral stereochemistry. Optical transmission spectra were recorded in the range 190–2100 nm and optical band gap energy was determined. The band gap energy (Eg) for all separated compounds lies in the range of semiconductors. On the other hand, the inhibition and adsorptive properties of the ligands for the corrosion of aluminum in 1 M HCl solutions were studied using traditional weight loss measurements. The results revealed that bis(2-methoxy-benzaldehyde)oxaloyldihydrazone has a greater inhibition than bis(2-hydroxy-1- naphthaldehyde)oxaloyldihydrazone. The adsorption of the inhibitors on metal surface was found to be spontaneous first order reaction and consistent well with the mechanism of physical adsorption. The adsorption data fitted well to Freundlich, Langmuir and Frumkin adsorption isotherms.
References
- M. Mohan, N. S. Gupta, M. P. Gupta, A. Kurnar, M. Kurnar, N. K. Jha, Inorg. Chim. Acta. 152, 25, (1988).
- P. Barbazan, R. Carballo, B. Covelo, C. Lodeiro, J. C. Lima, E. M. Vazquez-Lopez, Eur. J. Inorg. Chem. 2713 (2008).
- A. S. Pedrares, N. Camina, J. Romero, M. L. Duran, J. A. Garcia Vazquez, Polyhedron 27, 3391, (2008).
- E. M. Sherif, A. H. Ahmed, Synth. React. Inorg. Met-Org. Chem. 40, 365, (2010).
- M. Ghasemian, A. Kakanejadifard, F. Azarbani, A. Zabardasti, S. Shirali, Z. Saki, S. Kakanejadifard, Spectrochim. Acta A 38, 643, (2015).
- A. H. Ahmed, E. Ewais, J. Chem. Pharm. Res. 4, 3349 (2012).
- J. M. Price, Federation Proc. 20, 223, (1961).
- V. P. Singh, A. Katiyar, Pestic. Biochem. Phys. 92, 8, (2008).
- V. P. Singh, P. Singh, A. K. Singh, Inorg. Chim. Acta 379, 56, (2011).
- A. M. Hassan, A. H. Ahmed, H. A. Gumaa, B. H. Mohamed, A. M. Eraky, J. Chem. Pharm. Res. 7, 91 , (2015) and references therein.
- H. Abd El-Wahab, M. Abd El-Fattah, A. H. Ahmed, A. A. Elhenawy, N. A. Alian, J. Organomet. Chem. 791, 99, (2015).
- V. Lozan, P.-G. Lassahn, C. Zhang, B. Wu, C. Janiak, G. Rheinwald, H. Lang, Z. Naturforsch. B 58, 1152, (2003).
- W. H. Hegazy, Monatsh. Chem. 132, 639 (2001).
- R. Dinda, P. Sengupta, S. Ghosh, T. C. W. Mak, Inorg. Chem. 41, 1684, (2002).
- R. Raveendran, S. Pal, Polyhedron 24, 57, (2005).
- M. Salavati-Niasari, A. Sobhani, J. Mol. Catal. A 285, 58, (2008).
- R. A. Lal, S. Adhikari, A. Kumar, J. Chakraborty, S. Bhaumik, Synth. React. Inorg. Met.-Org. Chem. 32, 81 (2002).
- R. A. Lal, D. Basumatary, S. Adhikari, A. Kumar, Spectrochim. Acta A 69, 706, (2008).
- A. H. Ahmed, Rev. Inorg. Chem. 34, 153 (2014).
- A. H. Ahmed, M. S. Thabet, Syn. and Rea. in Ino. and Metal-Org. Chem. 45, 1632, (2015).
- A. H. Ahmed, A. G. Mostafa, Mater. Sci. Eng. C 29, 877, (2009).
- A. H. Ahmed, M. S. Thabet, J. Mol. Struct. 1006, 527, (2011).
- M. S. Thabet, A. H. Ahmed, J. Porous Mater. 20, 319, (2013).
- A. H. Ahmed, J. Mol. Struct. 839, 10, (2007).
- R. Lopez-Sesenes, J. G. Gonzalez-Rodriguez, M. Casales, L. Martinez, J. C. Sanchez-Ghenno, Int. J. Electrochem. Sci. 6, 1772, (2011).
- J. Aljourani, M. A. Golozar, K. Raeissi, Mater. Chem. Phys. 121, 320, (2010).
- H. S. Awad, S. A. Gawad, Anti Corros. Meth. Mater. 52, 328. (2005).
- E. W. Flick, Corrosion Inhibitors, Park Ridge, New Jersey, 1987.
- T. M. Salama, A. H. Ahmed, Z. M. El-Bahy, Micropor. Mesopor. Mater. 89, 251, (2006).
- M. M. Rashad, A. M. Hassan, A. M. Nassar, N. M. Ibrahim, A. Mourtada, Appl. Phys. A 117, 877, (2013).
- Tauc, J Mater. Res. Bull. 3, 37, (1968).
- K. Orubite-Okorosaye, N. C. Oforka, J. Appl. Sci. Environ.Mgt. 8, 57, (2004).
- I. A. Mohammed-Dabo, S. A.Yaro, G. Abubakar, S. I. Ayilara, T. U. Apugo-Nwosu, S. A. Akuso, J. Basic. Appl. Sci. Res. 1, 1989, (2011).
- R. A. Lal, S. Adhikari, A. Kumar, In. J. Chem. 36A, 1063, (1997).
- K. Burger, I. Ruff, F. Ruff, J. Inorg. Nucl. Chem. 27, 179, (1965).
- O. B. Chanu, A. Kumar, A. Ahmed, R. A. Lal, J. Mol. Struct. 1007, 257, (2012).
- A. M Ali, A. H Ahmed, T. A Mohamed, B. H Mohamed, Transit. Met. Chem. 32, 461, (2007).
- S. Salapathy, B. Sahoo, J. Inorg. Nucl. Chem. 32, 2223, (1970).
- A. H. Ahmed, A. M. Hassan, H. A. Gumaa, B. H. Mohamed, A. M. Eraky, Journal of Advances in Chemistry 11, 3834, (2015).
- D. Kumar, A. Syamal, L. K. Sharma, J. Coord. Chem. 61, 1788, (2008).
- A. Kumar, R. A. Lal, O. B. Chanu, R. Borthakur, A. Koch, A. Lemtur, S. Adhikari, S. Choudhury, J. Coord. Chem. 64, 1729, (2011).
- K. Nakamoto, Inorganic Spectra of Inorganic and Coordination Compounds, 2Ed, John Wiley & Sons, New York, 1970.
- C. Furlani, A. Furlani, J. Inorg. Nucl. Chem. 19, 51, (1961).
- F. A. Cotton, G. Wilkinson, Advanced Inorganic Chemistry, 2Ed, Interscience, 1966.
- A. B. P. Liver, Inorganic electronic spectroscopy, Elsevier, New York, 1984.
- D. Kivelson, R. Nieman, ESR Studies on the Bonding in Copper Complexes, J Chem. Phys. 35, 149, (1961).
- F. Karipcin, B. Dede, Y. Caglar, D. Hur, S. Ilican, M. Caglar, Y. Sahin, Opt. Commun. 272, 131, (2007).
- N. Turan, B. Gündüz, H. Körkoca, R. Adigüzel, N. Çolak, K. Buldurun, J. Mex. Chem. Soc. 58, 65, (2014).
- S. K. Sengupta, O. P. Pandey, B. K. Srivastava, V. Sharma, Transit. Met. Chem. 23, 349, (1998).
- M. L. Fu, G. C. Guo, X. Liu, L. Z. Cai, J. S. Huang, Inorg. Chem. Commun. 8, 18, (2005).
- P. C. Okafor, E. E. Ebenso, U. J. Ekpe, Int. J. Electrochem. Sci. 5, 978 (2010).
- M. Karakus, M. Sahin, S. Bilgic, Mater. Chem. Phys. 9, 561, (2005).
- J. Rodosevic, M. Kliskic, L. J. Aljinovic, S. Vuko,.In the Proceedings of the 8th European Symposium on Corrosion inhibition, Ann Univ. ferrara, Italy, 1995, pp: 817.
- R. Mohammad, M. Hadj, S. Mina, B. Pourya, J. Appl. Sci. Enviro. Sanit. 6, 1, (2011).
- A. L. Ahmad, S. Bhatia, N. Ibrahim, S. Sumathi, Braz. J. Chem. Eng. 22, 1, (2005).
- H. Shockry, M. Yuasa, I. Sekine, R. M. Issa, H.Y. El-baradie, G. K. Gomma, Corros. Sci. 40, 2173, (998).
- N. O. Eddy, P. Ekwumemgbo, S. A. Odoemelam, Int. J. Phys. Sci. 3, 1, (2008).
- H. Ashassi-Sorkhabi, B. Shaabani, B. Aligholipour, D. Seifzadeh, Appl. Surf. Sci. 252, 4039, (2006).
- S. Bilgic, M. Sahin, Mater. Chem. Phys. 70, 290, (2001).


