STUDY OF PHYSICAL AND CHEMICAL CHARACTERIZATION OF NANOCOMPOSITE POLYSTYRENE / GRAPHENE OXIDE HIGH ACIDITY CAN BE APPLIED IN THIN FILMS
- Nanocomposite,
- Dispersion,
- Graphene oxide,
- Polystyrene,
- Thin films
Copyright (c) 2017 Reza Jamshidi Rodbari, Rune Wendelbo, Lourdes Cristina Lucena Agostinho Jamshi, Eduardo Padrón Hernández, Luciano Nascimento

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
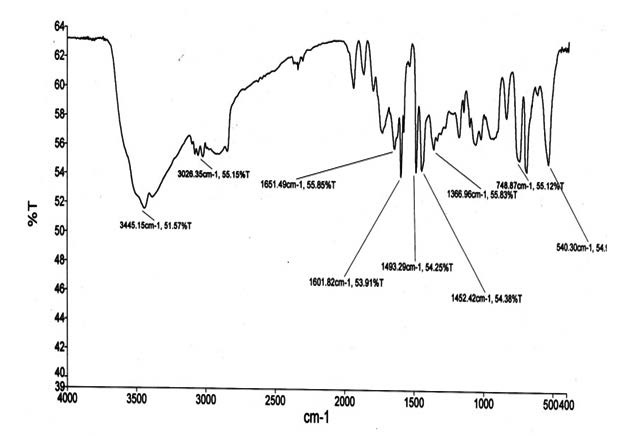
This paper shows a study of Nanocomposite formed by adding reduced Graphene oxide with high acidity and polystyrene. The interest and research in the material is due to the ability of these nanoparticles significantly altering the electrical and mechanical properties of the polymer, even addition of small levels. The existence of functional groups on the graphene oxide containing abundant oxygen such as; epoxy, hydroxyl and carboxylic acid, can be well dispersed in the polymer because of its good interaction with polymer chains. In this study we used the solution by dispersing method to that made the use of solvent tetrahydrofuran (THF), for purposes of obtaining a reaction with functionalization of graphene oxide / polystyrene in time of 48 hours. The analyses of physical-chemical characterizations were made diffraction X-ray (XRD), scanning electron microscopy (SEM), Infrared Spectroscopy (IRD), Thermogravimetric Analysis (TG) and Differential calorimeter by scanning (DSC). The results obtained by XRD diffraction pattern showed a strong expansion in the peak, indicating amorphization on single sheets of graphene oxide due to distorted sp3 sites CO. The morphology of the nanocomposite structure was with surface roughness, folds and rough predominant oxidation process of oxygenated functional groups. Their techniques showed the range of absorption, crystallinity degree and the mass loss. Finally, current and future possible applications of formed polystyrene nanocomposite/ graphene oxide show high acidity efficiency in the use of thin films.
References
- TZALENCHUK A.; LARA-AVILA S.; KALABOUKHOV, A;. PAOLILLO, S.M.; SYVAJARVI, R.; YAKIMOVA, O.; KAZAKOVA, T.; JANSSEN, V. FALKO, E.; KUBATKIN, S. Graphene: Setting New Standards, Nature Nanotechnology, 5, 186-189 (2010).
- J. C. MEYER, A. K. GEIM, M. I. KATSNELSON, K. S. NOVOSELOV E T.J. BOOTH, S. ROTH. The structure of suspended Graphene sheets, Nature, 446, 60-63 (2007).
- BLAKE, P., HILL, E. W., CASTRO NETO, A. H., NOVOSELOV, K. S., JIANG, D., YANG, R., BOOTH, T. J., GEIM, A. K. (2007) Making Graphene visible. Applied Physics Letters. 91:1-3.
- MA, P.C.; SIDDIQUI, N.; MAROM, G., KIM, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based Nanocomposites: A review. Composites Part A: Applied Science and Manufacturing, v. 41, p. 1345-1367, 2010.
- EDA, G., FANCHINI, G., & CHHOWALLA, M. (2008). Large-area ultrathin films of reduced in Graphene oxide as a transparent and flexible electronic material. Nat Nano, 3(5), 270-274.
- LAMBIN, P.; FINK, E J .Electronic states of carbon materials. Encyclopedia of Condensed Matter Physics 1. pp.142-151,2007.
- BLAKE, P., BRIMICOMBE, P. D., NAIR, R. R., BOOTH, T. J., JIANG, D., SCHEDIN, F., PONOMARENKO, L. A., MOROZOV, S. V., GLEESON, H. F., HILL, E. W., GEIM, A. K., NOVOSELOV, K. S.(2008) Graphene-Based Liquid Crystal Device. Nano Lett. 8:1704–1708.
- Y. ZHANG, Y. TAN, H. L. STORMER E P. KIM. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature, 438 201-204 (2005).
- PEI, Q. X.; ZHANG, Y. W.; SHENOY, V. B. A molecular dynamics study of the mechanical properties of hydrogen functionalized Graphene. Carbon, v. 48, n. 3, p. 898-904, 2010.
- BUENO, J.M .Propriedades Eletrônicas de Grafeno com Defeitos. Tese ( Doutorado no Departamento de Física)-João Pessoa-PB, Universidade Federal da Paraíba-UFPB,14p,2001.
- FISCHER, H. Polymer Nanocomposites: from fundamental research to specific applications. Materials Science and Engineering pp. 763- 772, 2003.
- TANAKA, T.; KOZAKO, M.; FUSE, N.; OHKI, Y.Proposal of a multi-core model for polymer Nanocomposite dielectrics.Transactions on Dielectrics and Electrical Insulation 12, pp. 669-681, 2005.
- HEO C; MOON H-G; YOON, C.S; CHANG, J-H: ABS Nanocomposite films based on functionalized-Graphene sheets. J App PolymSci.124. pp.4663–4670,2012.
- WHA-YONG LEE.; SEUNG-YEOL, YANG.; JIN-NAM, JEON.; YOUNG, JAE ,CHO.; YONGWOO, KWON.; YONG-SEOG, KIM. Ultra-thin Graphene Oxide/Polymer Multi-Layer Encapsulation for Flexible OLED. Displays.Dept. of Materials Science and Engineering,Hongik Univ., Seoul, Korea. SID 2014 DIGESTS.
- F.A, HE. H-J, WU. X-.L, YANG. K-H, LAM.; L-W,H,CHAN. Novel exfoliated graphite Nanoplates/syndiotactic polystyrene composites prepared by solution-blending, Polym. Test. 42 (2015) 45.


