CHARACTERIZATION OF POLYPHENOL PROFILE OF EXTRACTS OBTAINED FROM GRAPE POMACE AND SYNERGISTIC EFFECT OF THESE EXTRACTS AND FUNGICIDES AGAINST Botrytis cinerea

- Botrytis cinerea control,
- grape pomace extraction,
- synergism.
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Botrytis cinerea is a phytopathogenic fungus that infects the aerial parts of approximately 250 plant species. Control of this fungus is based on synthetic fungicide application. However, it has been reported the detection of isolates of B. cinerea resistant to all fungicides. Due to the emergence of resistant isolates of phytopathogenic fungi to commercial fungicides and the concomitant reduction in the efficacy of fungicides, various strategies have been developed to prevent or delay this problem. One of them is the use of synergistic mixtures among fungicides and plant extracts. In this work, extracts obtained from grape pomace of Syrah, Cinsault, or Cabernet Sauvignon varieties were analyzed. The phenolic composition in the fractions was different. Quercetin and kaempferol were observed in all fractions, in exception of Cinsault/chloroform fraction. Interestingly, gallic acid was found only in Cinsault extracts. The flavan-3-ols catechin and (-)-epicatechin are only found in Cinsault/ethyl acetate fraction.
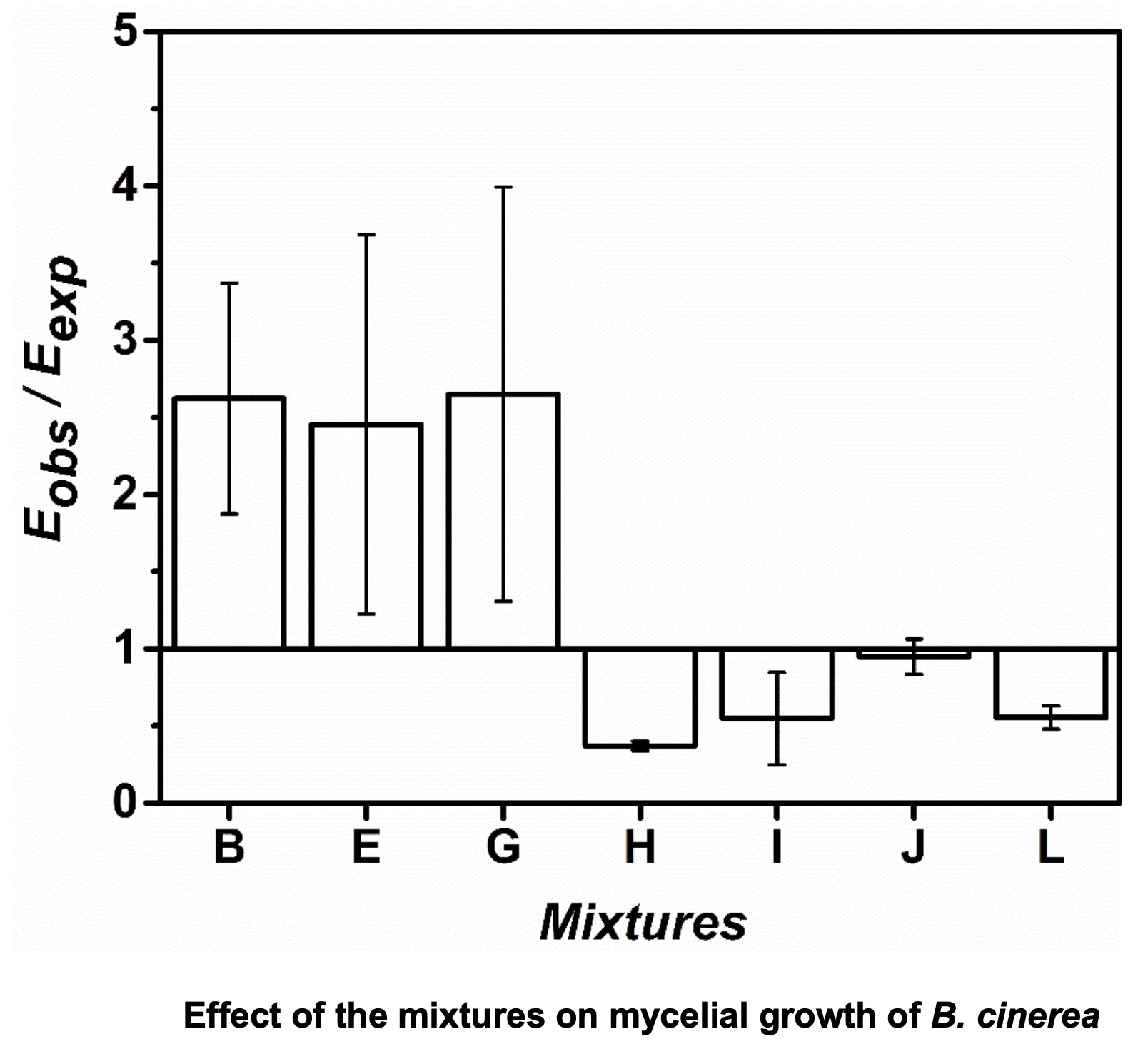
The effect of mixtures among these extracts and the fungicides Tercel and Cantus on mycelial growth of B. cinerea was analyzed. Mixtures of chloroform and ethyl acetate fractions from Cinsault grape pomace extracts and the fungicide Tercel at 0.1 ppm showed a synergistic interaction.
References
- Y. Elad, B. Williamson, P. Tudzynski, N. Delen. in Botrytis Biol. Pathol. Control, edited by Y. Elad, B. Williamson, P. Tudzynski, and N. Delen (Springer Netherlands, Dordrecht, 2007), pp. 1–8.
- M. Hahn. J. Chem. Biol. 7, 133, (2014).
- M. Esterio, G. Muñoz, C. Ramos, G. Cofré, R. Estévez, A. Salinas, J. Auger. Plant Dis. 95, 683, (2011).
- B.A. Latorre, R. Torres. Crop Prot. 40, 49, (2012).
- F. van den Bosch, N. Paveley, M. Shaw, P. Hobbelen, R. Oliver. Plant Pathol. 60, 597, (2011).
- F. Hossain, P. Follett, K. Dang Vu, M. Harich, S. Salmieri, M. Lacroix. Food Microbiol. 53, 24, (2016).
- B. Fielding, C.-L. Knowles, F. Vries, J. Klaasen. Agriculture 5, 400, (2015).
- J.H. Kim, B.C. Campbell, N. Mahoney, K.L. Chan, R.J. Molyneux, G.S. May. Lett. Appl. Microbiol. 45, 134, (2007).
- J.H. Kim, B.C. Campbell, N. Mahoney, K.L. Chan, R.J. Molyneux, G.S. May. FEMS Microbiol. Lett. 270, 284, (2007).
- J.H. Kim, B.C. Campbell, N. Mahoney, K.L. Chan, R.J. Molyneux. Mycopathologia 171, 291, (2011).
- V. Dzhavakhiya, L. Shcherbakova, Y. Semina, N. Zhemchuzhina, B. Campbell. Front. Microbiol. 3, 1, (2012).
- S.A. Zacchino, E. Butassi, M. Di Liberto, M. Raimondi, A. Postigo, M. Sortino. Phytomedicine 37, 27, (2017).
- D. Xu, G. Yu, P. Xi, X. Kong, Q. Wang, L. Gao, Z. Jiang. Molecules 23, 1455, (2018).
- L. Mendoza, K. Yañez, M. Vivanco, R. Melo, M. Cotoras. Ind. Crops Prod. 43, 360, (2013).
- A. Schieber, F.. Stintzing, R. Carle. Trends Food Sci. Technol. 12, 401, (2001).
- L. Mendoza, M. Cotoras, M. Vivanco, B. Matsuhiro, S. Torres, M.J. Aguirre. J. Chil. Chem. Soc. 58, 1725, (2013).
- M. Cotoras, H. Vivanco, R. Melo, M.J. Aguirre, E. Silva, L. Mendoza. Molecules 19, 21154, (2014).
- Q. Zhang, J. Zhang, J. Shen, A. Silva, D.A. Dennis, C.J. Barrow. J. Appl. Phycol. 18, 445, (2006).
- G. Muñoz, P. Hinrichsen, Y. Brygoo, T. Giraud. Mycol. Res. 106, 594, (2002).
- L. Mendoza, P. Espinoza, A. Urzúa, M. Vivanco, M. Cotoras. Molecules 14, 1966, (2009).
- Y. Levy, M. Benderly, Y. Cohen, U. Gisi, D. Bassand. EPPO Bull. 16, 651, (1986).
- K. Thevissen, F.R. Terras, W.F. Broekaert. Appl. Environ. Microbiol. 65, 5451, (1999).
- J. Morales, L. Mendoza, M. Cotoras. J. Appl. Microbiol. 123, 969, (2017).
- L. Sanhueza, M. Tello, M. Vivanco, L. Mendoza, M. Wilkens. Adv. Microbiol. 04, 225, (2014).

