Vol 61 No 3 (2016): Journal of the Chilean Chemical Society
Original Research Papers
A HIGHLY EFFICIENT SYNTHESIS OF SUBSTITUTED IMIDAZOLES VIA A ONE-POT MULTICOMPONENT REACTION BY USING UREA/HYDROGEN PEROXIDE (UHP)
Published
September 10, 2016
Keywords
- Multicomponent reaction,
- Imidazole,
- Benzil,
- Urea,
- Hydrogen peroxide
How to Cite
Maleki, A., & Alirezvani, Z. (2016). A HIGHLY EFFICIENT SYNTHESIS OF SUBSTITUTED IMIDAZOLES VIA A ONE-POT MULTICOMPONENT REACTION BY USING UREA/HYDROGEN PEROXIDE (UHP). Journal of the Chilean Chemical Society, 61(3). Retrieved from https://jcchems.com/index.php/JCCHEMS/article/view/90
Copyright (c) 2017 Ali Maleki, Zahra Alirezvani

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
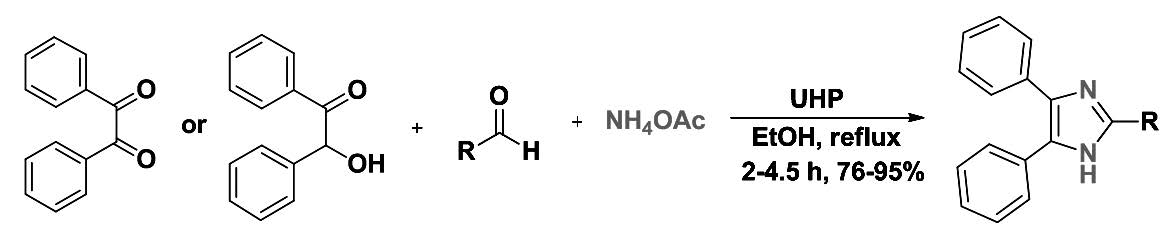
A simple, versatile and highly efficient synthesis of 2,4,5-trisubstituted imidazoles by a condensation reaction of benzil or benzoin, aldehydes and ammonium acetate in the presence of urea/hydrogen peroxide (UHP) as a mild and efficient molecular catalyst in refluxing ethanol is described. The present methodology offers several advantages such as green protocol, inexpensive catalyst, high-to-excellent yields, and simple and easy work-up procedure.
References
- A. Dömling, W. Wang, K. Wang, Chem. Rev. 112, 3083, (2012).
- M. M. Heravi, M. Daraie, V. Zadsirjan, Mol. Divers. 19, 577, (2015); and the references cited therein.
- A. D. Palkowitz, M. I. Steinberg, K. J. Thrasher, J. K. Reel, K. L. Hauser, K. M. Zimmerman, S. A. Wiest, C. A. Whitesitt, R. L. Simon, J. Med. Chem. 37, 4508, (1994).
- J. I. Trujillo, J. R. Kiefer, W. Huang, A. Thorarensen, L. Xing, N. L. Caspers, J. E. Day, K. J. Mathis, K. K. Kretzmer, B. A. Reitz, Bioorg. Med. Chem. Lett. 19, 908, (2009).
- L. L. Chang, K. L. Sidler, M. A. Cascieri, S. de Laszlo, G. Koch, B. Li, M. MacCoss, N. Mantlo, S. O’Keefe, M. Pang, A. Rolando, W. K. Hagmann, Bioorg. Med. Chem. Lett. 11, 2549, (2011).
- D. Sharma, B. Narasimhan, P. Kumar, V. Judge, R. Narang, E. De Clercq, J. Balzarini, Eur. J. Med. Chem. 44, 2347, (2009).
- S. Kumar, J. Boehm, J. C. Lee, Nature Rev. Drug Disc. 2, 717, (2003).
- A. J. King, D. R. Patrick, R. S. Batorsky, M. L. Ho, H. T. Do, S. Y. Zhang, R. Kumar, D. W. Rusnak, A. K. Takle, D. M. Wilson, Cancer Res. 66, 11100, (2006).
- (a) A. Maleki, Tetrahedron 68, 7827, (2012). (b) A. Maleki, Tetrahedron Lett. 54, 2055, (2013). (c) A. Maleki, Helv. Chim. Acta. 97, 587, (2014). (d) A. Maleki, N. Ghamari, M. Kamalzare, RSC Adv. 4, 9416, (2014). (e) A. Maleki, M. Kamalzare, Catal. Commun. 53, 67, (2014). (f) A. Maleki, M. Kamalzare, Tetrahedron Lett. 55, 6931, (2014). (g) A. Maleki, R. Paydar, RSC Adv. 5, 33177, (2015). (h) A. Maleki, Z. Alrezvani, S. Maleki, Catal. Commun. 69, 29, (2015). (i) A. Maleki, H. Movahed, R. Paydar, RSC Adv. 6, 13657, (2016).


