Vol 61 No 3 (2016): Journal of the Chilean Chemical Society
Original Research Papers
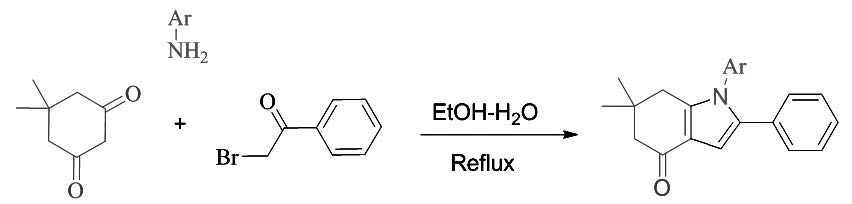
SYNTHESIS OF 1-ARYL-6,6-DIMETHYL-2-PHENYL-6,7-DIHYDRO-1H-INDOL-4(5H)-ONES BY TWO STEPS, IN A THREE-COMPONENT REACTION
Published
September 10, 2016
Keywords
- Phenacyl bromide,
- Dimedone,
- Anilines,
- 1H-Indol-4(5H)-ones,
- Three-component reaction
How to Cite
Khalafy, J., Badparvar, F., & Marjani, A. P. (2016). SYNTHESIS OF 1-ARYL-6,6-DIMETHYL-2-PHENYL-6,7-DIHYDRO-1H-INDOL-4(5H)-ONES BY TWO STEPS, IN A THREE-COMPONENT REACTION. Journal of the Chilean Chemical Society, 61(3). Retrieved from https://jcchems.com/index.php/JCCHEMS/article/view/89
Copyright (c) 2017 Jabbar Khalafy, Fahimeh Badparvar, Ahmad Poursattar Marjani

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
An efficient method for the synthesis of 1-aryl-6,6-dimethyl-2-phenyl-6,7-dihydro-1H-indol-4(5H)-ones is achieved in two steps, using a three-component reaction of phenacyl bromide, dimedone and aniline derivatives in water-ethanol (1:1) under reflux conditions.
References
- N.K. Kaushik, N. Kaushik, P. Attri, N. Kumar, C.H. Kim, A.K. Verma, E.H. Choi, Molecules. 18, 6620 (2013).
- R. Dhani, A. Avinash, S.K. Salenaagina, M.V.S. Teja, P. Masthanaiah, P.R. Rathnam, V.C. Silpa, J. Chem. Pharm. Res. 519 (2011).
- H.B. Broughton, H.J. Bryant, M.S. Chambers, N.R. Curtis, WO 9962, 899, 1999; Chem. Abstr. 1999, 132: 12259y.
- D.B. Fick, M.M. Foreman, A.J. Glasky, D.R. Helton, WO; 0311, 396, 2003; Chem. Abstr. 2003, 138: 163572v.
- S. Suzen, E. Buyukbingol, Il Farmaco. 55, 246 (2000).
- E. Buyukbingol, S. Suzen, G. Klopman, IlFarmaco. 49, 443 (1994).
- C.C. Chiang, Y.H. Lin, S.F. Lin, C.L. Lai, C. Liu, W.Y. Wei, S.C. Yang, R.W. Wang, L.W. Teng, S.H. Chuang, J.M. Chang, T.T. Yuan, Y.S. Lee, P. Chen, W.K. Chi, J. Y. Yang, H.J. Huang, C.B. Liao, J.J. Huang, J. Med. Chem. 53, 5929 (2010).
- P.M. Lieberman, A. Wolfler, P. Felsner, D. Hofer, K. Schauenstien, Arch. Allergy Immunol. 112, 203 (1997).
- S. Suzen, E. Buyukbingol, IlFarmaco. 53, 525 (1998).
- C.W. Zapf, J.D. Bloom, J.L. McBean, R.G. Dushin, T. Nittoli, C. Ingalls, A.G. Sutherland, J.P. Sonye, C.N. Eid, J. Golas, H. Liu, F. Boschelli, Y. Hu, E. Vogan, J.I. Levin, Bioorg. Med. Chem. Lett. 21, 2278 (2011).
- K. Spyridonidou, M. Fousteris, M. Antonia, A. Chatzianastasiou, A. Papapetropoulos, S. Nikolaropoulos, Bioorg. Med. Chem. Lett. 19, 4810 (2009).
- (a) M. Bandini, A. Melloni, A. Umani-Ronchi, Angew. Chem., Int. Ed. 43, 550 (2004). (b) J.F. Austin, D.W.C. MacMillan, J. Am. Chem. Soc. 124, 1172 (2002). (c) K.B. Jensen, J. Thorhange, R.G. Hazel, K.A. Jorgensen, Angew. Chem., Int. Ed. 40, 160 (2001).
- (a) N. Srivastava, B.K. Banik, J. Org. Chem. 68, 2109 (2003). (b) G. Bartoli, M. Bartolacci, M. Bosco, G. Foglia, A. Giuliani, E. Marcantoni, L. Sambri, E. Torregiani, J. Org. Chem. 68, 4594 (2003).
- (a) N. Yoshiaki, Y. Masato, I. Youichi, H. Masnobu, U. Sakae, J. Am. Chem. Soc. 124, 11846 (2002). (b) E. Wenkert, E.C. Angell, V.F. Ferreira, E.L. Michelotti, S.R. Piettre, I. Chen, S. Safe, L. Bjeldanes, Biochem. Pharmacol. 51, 1069 (1996).
- P. Ashok Gajapathi Raju, R. Mallikarjunarao, K. Venu Gopal, J. Sreeramulu, D. Maheswara Reddy, K.P. Krishnamurthi, S. Rajasekhar Reddy, J. Chem. Pharm. Res. 5, 21 (2013).
- P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice. Oxford University Press, New York, 1998.
- D. Warren, Green Chemistry. A Teaching Resource. Royal Society of Chemistry, Cambridge, 2001.
- J. Clark, D. Macquarrie, Handbook of Green Chemistry and Technology. Blackwell Publishing, Abingdon, Oxfordshire, 2002.
- J. Sapi, J.Y. Laronze, Arkivoc. v, 208 (2004).
- M. E. Huwaida Hassaneen, Arkivoc. i, 154 (2007).
- For a monograph on MCRs, see: Multicomponent Reactions, ed. J. Zhu, H. Bienayme´, Wiley-VCH, 2005.
- Symposium in print on MCRs, see: Tetrahedron Symposia-in-Print, ed. I. Marek, 2005, vol. 67, p. 11299.
- H. Batchu, S. Batra. Eur. J. Org. Chem., 2935 (2012).
- S. Kaladevi, J. Sridhar, B. Abhilashamole, S. Muthusubramanian, N. Bhuvanesh, RSC Adv. 4, 34382 (2014).
- J. Khalafy, N. Etivand, S. Dilmaghani, M. Ezzati, A. Poursattar Marjani, Tetrahedron Lett. 55, 3781 (2014). b) J. Khalafy, A. Poursattar Marjani, F. Salami, Tetrahedron Lett. 55, 6671 (2014). c) J. Khalafy, M. Ezzati, M. Rimaz, A. Poursattar Marjani, H. Yaghoobnejad Asl, J. Iran. Chem. Soc. 11, 1067 (2014). d) J. Khalafy, M. Mohammadlou, M. Mahmoody, F. Salami, A. Poursattar Marjani, Tetrahedron Lett. 56, 1528 (2015). e) A. Poursattar Marjani, J. Khalafy, F. Salami, M. Mohammadlou, Synthesis. 47, 1656 (2015). f) A. Poursattar Marjani, J. Khalafy, F. Salami, M. Ezzati, Arkivoc. v, 277 (2015). g) A. Poursattar Marjani, J. Khalafy, S. Mahmoodi. Arkivoc. iii, 262 (2016). h) A. Poursattar Marjani, J. Khalafy, A. Rostampoor. J. Heterocycl. Chem., in press (2016). http://onlinelibrary. wiley.com/doi/10.1002/jhet.2637/full.
- K. Nagarajan, J. David, R.K. Shah, J. Med. Chem. 19, 508 (1976).
- R. Mallikarjuna Rao, G. Nagaraja Reddy, J. Sreeramulu, Der Pharma Chemica. 3, 301 (2011).
- P.N. Maini, M.P. Sammes, A.R. Katritzky, J. Chem. Soc., Perkin Trans. 1. 161 (1988).
- S.R. Ramadas, S. Padmanabhan, J. Prakt. Chem. 320, 863 (1978).
- P.B. Terentiev, Y.G. Boundel, A.N. Kost, B.I. Maksimov, J. Heterocyclic. Chem. 19, 645 (1982).


