- N-propylaminepolyaniline,
- Direct oxidation,
- Friedel-Craft synthesis
Copyright (c) 2017 M. A. Takassi, A. Zadehnazari

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
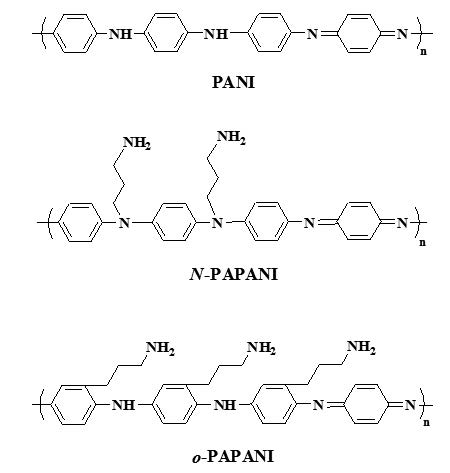
In present investigation, N-substituted propylaminepolyaniline (N-PAPANI) was prepared by reaction of dimethylsulfoxide solution of polyaniline (emeraldine) base with sodium hydride, then reacted with 3-chloropropylaminehydrochloride. The N-PAPANI was characterized using reflectance Fourier transformation infrared (FTIR), cyclic voltammetry, UV-vis spectroscopy and chemical analysis. Polyaniline (PANI) was also subjected to a reaction with 3-chloropropylaminehydrochloride and ferric chloride in acetonitrile as solvent under refluxing temperature (85-90 ºC) (Friedel-Craft synthetic method) to produce ring substituted functionalized PAPANI. Characterization of PAPANI was carried out using various aforementioned techniques. PANI and N-PAPANI polymers exhibited strong UV-vis absorption maxima at 273-294 nm in solution. Cyclic voltammograms of the polymers on an indium-tin oxide (ITO)-coated glass substrate exhibited one pair of reversible redox couples at half-wave oxidation potentials (E1/2) around 0.84-0.86 V and 0.88 V, respectively, versus Ag/AgCl in an acetonitrile solution. The obtained polymers showed good solubility in common organic solvents such as tetrahydrofuran and chloroform compared with emeraldine which is difficult to process due to lack of solubility. Furthermore, these polymers are air stable.
References
- A.T. Lawal, G.G. Wallace, Talanta 119, 133, (2014).
- U. Lange, V.M. Mirsky, Anal. Chim. Acta 687, 105, (2011).
- M. Ates, Mater. Sci. Eng. C 33, 1853, (2013).
- A. Ramanavicius, Y. Oztekin, Z. Balevicius, A. Kausaite-Mikstimiene, V. Krikstolaityte, I. Baleviciute, V. Ratautaite, A. Ramanaviciene, Procedia Eng. 47, 825, (2012).
- N.K. Guimard, N. Gomez, C.E. Schmidt, Prog. Polym. Sci. 32, 921, (2007).
- J. Alam, L.A. Dass, M.S. Alhoshan, A.W. Mohammad, Adv. Polym. Technol. 32, E189, (2013).
- A.J. Heeger, Angew. Chem. Int. Ed 40, 2591, (2001).
- C.O. Sanchez, A. Isla, C. Bustos, F. Diaz, N. Gatica, J. Chil. Chem. Soc. 55, 233, (2010).
- E. Ortega, F. Armijo, I. Jessop, M.A. Del Valle, F.R. Diaz, J. Chil. Chem. Soc. 58, 1959, (2013).
- M.A. Takassi, M.R. Shishesaz, Polym. Res. J. 7, 129, (2013).
- J. Longun, J.O. Iroh, J. Appl. Polym. Sci. 128, 1425, (2013).
- A.G. Yavuz, A. Uygun, H. Kaplan Can, Carbohydr. Res. 346, 2063, (2011).
- H. Hasbullah, S. Kumbharkar, A.F. Ismail, K. Li, J. Membr. Sci. 397-398, 38, (2012).
- M.K. Kim, K. Shanmuga Sundaram, G. Anantha Iyengar, K.-P. Lee, Chem. Eng. J. 267, 51, (2015).
- C. Li, S. Mu, Synth. Met. 144, 143, (2004).
- J. Wang, D. Zhang, Adv. Polym. Technol. 32, E323, (2013).
- S.D. Shinde, M. Jayakannan, J. Appl. Polym. Sci. 127, 1781, (2013).
- E.-M. Kim, C.-K. Jung, E.-Y. Choi, C. Gao, S.-W. Kim, S.-H. Lee, O.P. Kwon, Polymer 52, 4451, (2011).
- J.-C. Chiang, A.G. MacDiarmid, Synth. Met. 13, 193, (1986).
- E. Shoji, M.S. Freund, Langmuir 17, 7183, (2001).
- S.-A. Chen, G.-W. Hwang, J. Am. Chem. Soc. 116, 7939, (1994).
- F. Wudl, R.O. Angus, F.L. Lu, P.M. Allemand, D. Vachon, M. Nowak, Z.X. Liu, H. Schaffer, A.J. Heeger, J. Am. Chem. Soc. 109, 3677, (1987).
- H. Tang, A. Kitani, T. Yamashita, S. Ito, Synth. Met. 96, 43, (1998).
- R.M. Silverstein, G.C. Bassler, T.C. Morrill Spectrometric Identification of Organic Compounds, 4th Ed. John Wiley & Sons, 1981; pp. 128.
- K. Nakanishi, P.H. Solomon Infrared Absorption Spectroscopy, 2nd ed.; Nankodo, Tokyo, 1977; pp. 33.


