- Antifungal Activity,
- Diarylhydrazones,
- Schiff bases
Copyright (c) 2017 Sergio Ortiz, Ronald Nelson, Víctor Kesternich, Marcia Pérez-Fehrmann, Philippe Christen, Laurence Marcourt

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
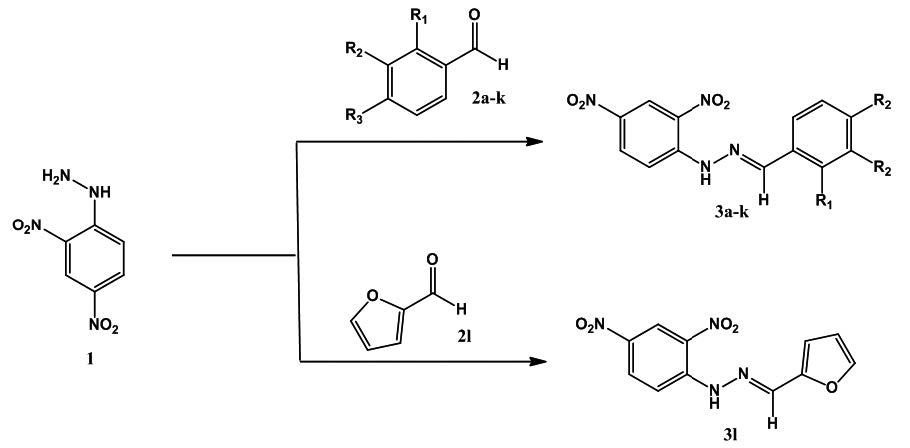
A new series of diarylhydrazones derived from 2,4-dinitrophenylhydrazine were synthesized via condensation with aromatic aldehydes whose structures have been determined by mass spectrometry, infrared spectroscopy, and nuclear magnetic resonance spectra. Yields were 50–99%. All compounds were screened in vitro for their antifungal activity. Preliminary results indicated that compounds 3g and 3h exhibited promising antifungal potency. Understanding of the structure of these compounds establishes a preliminary structure-activity relationship to form the basis of further investigation.
References
- Regina G. L., Coluccia A., Piscitelli F., Bergamini A., Sinistro A., Cavazza A., Maga G., Samuele A., Zanoli S., Novellino E., Artico M., Silvestri R. J. Med. Chem. 2007, 50, 5034.
- Regina G.L., Sarkar T., Bai R., Edler M. C., Saletti R., Coluccia A., Piscitelli F., Minelli L., Gatti V., Mazzoccoli C., Palermo V., Mazzoni C., Falcone C., Scovassi A. I., Giansanti V., Campiglia P., Porta A., Maresca B., Hamel E., Brancale A.. Novellino E., Silvestri R. J. Med. Chem. 2009, 52, 7512.
- Silvestri R., Cascio M. G., La Regina G., Piscitelli F., Lavecchia A., Brizzi A., Pasquini S., Botta M., Novellino E., Di Marzo V., Corelli F. J. Med. Chem. 2008, 51, 1560.
- Colak M., Demirel N. Tetrahedron: Asymmetry 2008, 19, 635.
- Yoshikawa N., Shibasaki M. Tetrahedron 2002, 58, 8289.
- Radecka-Paryzek W., Patroniak V., Lisowski J. Coord. Chem. Rev. 2005, 249, 2156.
- Gupta K. C., Sutar A. K., Lin C-C. Coord. Chem. Rev. 2009, 253, 1926.
- Mobinikhaledi A., Jabbarpour M., Hamta A. J. Chil. Chem. Soc. 2011, 56, 812.
- Rollas S., Küçükgüzel S. G. Molecules 2007, 12, 1910.
- Cheng L., Tang J-J., Luo H., Jin X-L., Dai F., Yang J., Qian Y-P., Li X-Z., Zhou B. Bioorg. Med. Chem. Lett. 2010, 20, 2417.
- Török, B., Sood, A., Bag S., Tulsan R., Ghosh S., Borkin D., Kennedy A. R., Melanson M., Madden R., Zhou W., LeVine H., Török M. Biochemistry. 2013, 52, 1137.
- Vicini, P., Zani, F., Cozzini, P., Doytchinova, I. Eur. J. Med. Chem. 2002, 37, 553.
- Küçükgüzel Ş. G,, Mazi A., Şahin F., Öztürk S., Stables J. P. Eur. J. Med. Chem. 2003, 38, 1005.
- Loncle C., Brunel J., Vidal N., Dherbomez M., Letourneux Y. Eur. J. Med. Chem. 2004, 39, 1067.
- Fortún J., Medicine, 1998, 91, 4231.
- Altıntop M. D., Özdemir A., Turan-Zitouni G., Ilgın S., Atlı Ö., Demirci F., Kaplancıklı Z. A. Molecules 2014, 19, 14809.
- Moran G. P., Sullivan D. J., Henman M. C., Creary C. E. M. C., Harrington B. J., Shanley D. B., Coleman D. C. Antimicrob Agents Chemother. 1997, 41, 617.


