UV SPECTROPHOTOMETRIC DETERMINATION OF pK’s OF 1,2,4-TRIAZOLINE-3-THIONES IN SODIUM HYDROXIDE SOLUTIONS
- Dissociation constants,
- CVA method,
- 1,
- 2,
- 4-triazoline-3-thiones
- UV ...More
Copyright (c) 2017 Vesna Dimova, Igor Jordanov, Lazo Dimitrov

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
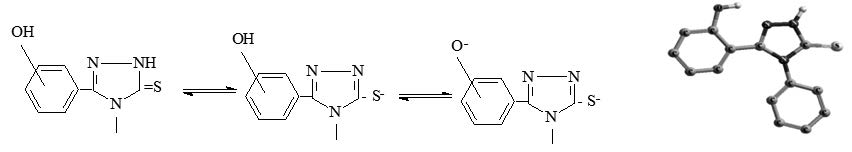
Using UV spectroscopy the dissociation constants of previously synthesized 4,5-disupstituted-1,2,4-triazoline-3-thiones are determinate. The UV spectra were recorded in aqueous sodium hydroxide solutions at different pH values in the range from 190 to 360 nm, immediately after preparation of the suspension. The pKTH values were obtained experimentally, graphically and by the Characteristic Vector Analysis method. Results show that the investigated compounds have two dissociation processes. The first process belongs to the dissociation of SH proton, while the second dissociation process is dissociation of OH proton.
References
- A. Mumtaz, A. Saeed, I. Malik, J. Chem. Soc. Pak., 36, 852 (2014).
- E. Düğdü, Y. Ünver, D. Ünlüer, K. Sancak, Molecules, 19, 2199 (2014).
- S. Maddila, M. Momin, S. Gorle, L. Palakondu, S. B. Jonnalagadda, J. Chil. Chem. Soc., 60, 2919, (2015).
- R. Katritzky, C. W. Rees, Comprehensive Heterocyclic Chemistry-Five-membered Rings with Three or more Heteroatoms, each with their Fused Carbocyclic Derivatives, Pergamon Press LTD, London, UK, 1984.
- A. F. Al-Ghamdi1, M. R. Aouad, Int. J. Electrochem. Sci., 8, 11248, (2013).
- M. S. Karthikeyan, D. J. Prasad, B. Poojary, K. S. Bhat, B. S. Holla, N. S. Kumari, Bioorg. Med. Chem., 14, 7482, (2006).
- Q. J. Xu, C. X. Li, G. D. Zhou, L. J. Zhu, C. J. Lin, Acta Phys.-Chim. Sin., 25, 86 (2009).
- Q. J. Xu, Z. Y. Wan, R. H. Yin, L. J. Zhu, W. M. Cao, G. D. Zhou, C. J. Lin, Acta Phys.-Chim. Sin., 24, 115 (2008).
- V. Dimova, N. Perisic-Janjic, Maced. J. Chem. Chem. Eng., 31, 89 (2012).
- V. Dimova, Turk. J. Chem., 35, 109 (2011).
- V. Dimova, Zaštita materijala, 54, 161 (2013).
- T. Eicher, S. Hauptmann, The Chemistry of Cheterocycles, Structure, Reactions, Synthesis and Application, Second, Completely Revised and Enlarged Edition, WILEY – VCH GmbH & Co. KGaA, Weinheim, 2003.
- C. H. Rochester, Acidity Functions, Academic Press, New York, 1971.
- A. Thakur, ARKIVOC, XIV, 49 (2005).
- L. Arman-Zubic, N. Perisic-Janjic, M. Lazarevic, J. Serb. Chem. Soc., 65, 619 (2000).
- J. T. Edward, I. Lantos, G. D. Derdall, S. C. Wong, Can. J. Chem., 55, 812 (1977).
- C. Tissier, M. Tissier, Bull. Soc. Chim. Fr., 2109 (1972).
- J. L. Simonds, J. Opt. Soc. Am., 53, 968 (1963).
- M. Lazarevic, V. Dimova, J. Csanádi, M. Popsavin, Lj. Klisarova, Bull. Chem. Technol. Macedonia, 16, 97 (1997).
- C. A. Tsoleridis, D. A, Charistos, G. V. Vagenas, J. Heterocyclic Chem., 34, 1715 (1997).
- S. Rollas, N. Kalyoncuğlu, D. Sur-Altiner, Y. Yeğenğlu, Pharmazie, 48, 308 (1993).
- A. Albert, E. P. Serjeant, The Determination of Ionization constants-A laboratory manual, Chapman and Hal, 2d edition, London and New York, 1971.
- R. A. Cox, K. Yates, J. Am. Chem. Soc, 100, 3861 (1978).


