CHIRAL CHROMATOGRAPHIC ANALYSIS AND ABSOLUTE STEREOCHEMISTRY OF RACEMIC CHLORPHENERAMINE USING CHIRAL HPLC-CD-OR AND TD-DFT CALCULATIONS
- HPLC-CD-OR,
- circular dichroism detection,
- optical rotation detection,
- DFT calculations
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
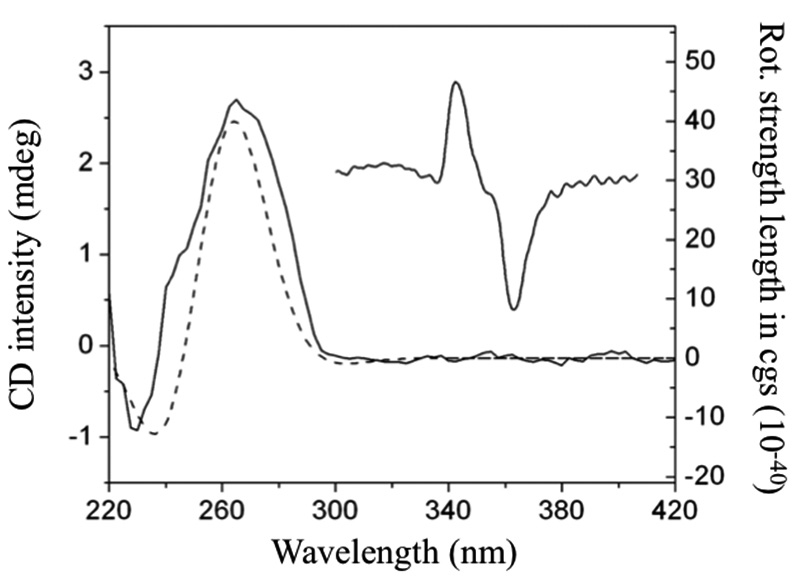
A chiral HPLC analysis combined with time-dependent density functional theory (TD-DFT) calculations was used for the assignment of the absolute configuration (AC) of both enantiomers of the antihistamine drug chlorpheniramine. Circular dichroism and optical rotation detections coupled to the chromatographic system (HPLC-CD-OR) permitted the on-line measurement of the CD spectra and [α]670 values, while TD-DFT at the B3LYP/TZVP//B3LYP/VDZ(P) and B3LYP/ aug-cc-pVDZ//B3LYP/VDZ(P) levels of theory for CD and OR, respectively, delivered theoretical chiroptical properties in the gas and solution states. Both calculations were consistent in predicting the correct combination of CD spectra and [α]670 sign and therefore permitted the absolute configuration (AC) assignment of the optical isomers. The AC proposed using this methodology agreed with a previous X-ray study.
References
- Lemke T.L.; Williams D.A. Foye´s Principles of Medicinal Chemistry. Philadelphia: Lippincott Williams & Wilkins; 2008. 38-43.
- H.D. Flack, G. Bernardinelli, Chirality 20, 681-690, (2008).
- G.H. Wagnière, On the interaction of light with molecules: pathways to the theoretical interpretation of chiroptical phenomena, in: N. Berova, P.L. Polavarapu, K. Nakanishi, R.W. Woody (Eds.), Comprehensive Chiroptical Spectroscopy Vol. 1, John Wiley & Sons, New York, 2012, pp. 3-34.
- L. Kott, W.B. Holzheuer, M.M. Wong, G.K. Webster, J. Pharm. Biomed. Anal. 43, 57-65, (2007).
- P.J. Stephens, J.J. Pan, F.J. Devlin, K. Krohn, T. Kurta´n, J. Org. Chem. 72, 3521-3526, (2007).
- P.J. Stephens, D.M. McCann, F.J. Devlin, A.B. Smith III, J. Nat. Prod. 69, 1055-1064, (2006).
- J.P. Castillo-González, N.I. González-Peña, M.A. Muñoz, J. Chil. Chem. Soc. 60, 2642-2644. (2016).
- M.A. Muñoz, N. González, P. Joseph-Nathan, J. Sep. Sci. 39, 2720- 2727. (2016).
- British Pharmacopoeia Volume I & II, Monographs: Medicinal and Pharmaceutical Substances, Ph. Eur. monograph 0386.
- M.N.G. James, G.J.B. Williams, Can. J. Chem., 1974, 52, 1872-1879.
- C. Bertucci, D. Tedesco, J. Chromatogr. A 1269, 69-81, (2012).
- P.D. Rice, Y.Y. Shao, S.R. Erskine, T.G. Teague, D.R. Bobbitt, Talanta 36, 473-478, (1989).
- S. Sinnecker, A. Rajendran, A. Klamt, M. Diedenhofen, F. Neese, J. Phys. Chem. A. 110, 2235-2245, (2006).
- T. Bruhn, A. Schaumlöffel, Y. Hemberger, G. Bringmann, SpecDis version 1.62, University of Wuerzburg, Germany, 2014. (http://www-organik.chemie.uni-wuerzburg.de/lehrstuehlearbeitskreise/bringmann/ specdis/).
- F. Neese, WIREs Comput. Mol. Sci. 2, 73-78, (2012).
- M. Valiev, E.J. Bylaska, N. Govind, K. Kowalski, T.P. Straatsma, H.J.J. van Dam, D. Wang, J. Nieplocha, E. Apra, T.L. Windus, W.A. de Jong, Comput. Phys. Commun. 181, 1477-1489, (2010).
- Lux Columns Complete Chiral Solutions Catalogue, Phenomenex, Torrance, CA, USA (2013).
- P.J. Stephens, F.J. Devlin, J.R. Cheeseman, M.J. Frisch, Chirality, 2002, 14, 288-296.
- J. Autschbach, S. Patchkovskii, T. Ziegler, S.J.A. van Gisbergen, E.J. Baerends, J. Chem. Phys., 2002, 117, 581-592.
- P.J. Stephens, J.-J. Pan, F.J. Devlin, M. Urbanová, J. Hájíček, J. Org. Chem., 2007, 72, 2508-2524.
- M. Kwit, J. Gawronski, L. Sbircea, N.D. Sharma, M. Kaik, D.R. Boyd, Chirality, 2009, 21, E37-E47.
- P.L. Polavarapu, N. Jeirath, T. Kurtán, G. Pescitelli, K. Krohn, Chirality, 2009, 21, E202-E207.
- P.J. Stephens, J.J. Pan, F.J. Devlin, J.R. Cheeseman, J. Nat. Prod., 2008, 71, 285-288.
- M.A. Muñoz, M.A. Bucio, P. Joseph-Nathan, Nat. Prod. Commun., 2013, 8, 1075-1078.
- E. Giorgio, N. Parrinello, S. Caccamese, C. Rosini, Org. Biomol. Chem., 2004, 2, 3602-3607.


