- risperidone,
- stability- indicating method,
- LC,
- tablets,
- stress testing
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
A stability- indicating LC method was developed and validated for the determination of risperidone in tablets.
Quantitation was achieved by LC/DAD at 294 nm over the concentration range of 25.00 μg/mL to 250.00 μg/mL. Mobile phase was a mixture of water: glacial acetic acid 0.50 %: triethylamine 0.80 %: acetonitrile (65.00: 0.32: 0.52: 34.16, v/v), using a Purosphere STAR RP-18e 250 x 4.5 mm (5μ) column, and paroxetine as internal standard.
The method exhibited an adequate linearity (r = 0.999), selectivity, precision (RSD ≤ 0.847 %) and accuracy (recoveries from 99.55 % to 101.35 %).
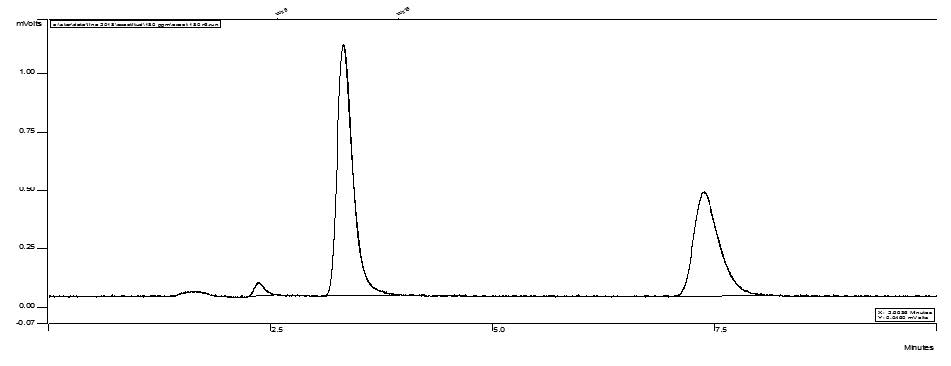
Risperidone was subjected to the stress conditions of oxidative, acid, base, thermal and photolytic degradation. Risperidone was found no degrade in basic or acid stress conditions, neither in thermal stress exposition (50, 70 and 100 ºC) no at visible or UV stress conditions, during the time of the study. Only two degradation products were observed with peroxide oxidation, well resolved from analyte peak, proving the stability-indicating power of the method.
The proposed method was found to be suitable for quantitative determination and the stability studies of risperidone in tablets.
References
- A. Gennaro, A. Remington: the Science and Practice of Pharmacy, Lippincott Williams /Wilkins, Philadelphia, 2000.
- The United States Pharmacopeia/ The National Formulary (USP 308). United States Pharmacopeial Convection Inc., Rockville, 2015.
- W. Lund. The Pharmaceutical Codex. The Pharmaceutical Press, London, 1994.
- S. Yoshioka, V. Stella. Stability of Drugs and Dosage Forms. Kluwer Academic Plenum Publishers, New York, 2000.
- G. Mc Evoy. AHFS Drug Information. American Society of Health- System Pharmacists, 2015.
- S. Sweetman. Martindale, Guía Completa de Consulta Farmacoterapéutica. Pharma Editores S.L., Barcelona, 2008.
- J. Hardman, L. Limbird. Las Bases Farmacológicas de la Terapéutica. Mc Graw-Hill, Mexico, 2006.
- S. Rajesh, T. Tomar, J. Joseph, A. Murthy, D. Yadav, G. Subbaiah, J. Pharm. Anal., 36, 231-235, (2004).
- A.E. Zeinab, B. El-Zeany, M. El-Houssini, J. Pharm. Anal.,.36, 975-981, (2005).
- D. Sharma, K.S. Srinivas, P. Gupta, D.P. Dwivedi, H. Dureja, M. Nagpal, P. Rakha, S. Goyal, Acta Pharmaceutica Sciencia, 52, 345-352, (2010).
- Z.R. Dedania, R. Dedania, N.R. Seth J.B. Patel, Int J Anal Chem., 124917, (2011).
- S.L. Baldania K. Bhatt, R.S. Mehta, D.A. Sha, Indian J. Pharm. Sci., 70, 494-497, (2008).
- S. Ashour, N. Kattan, Int.J. Biomed. Sci., 9, 91-97, (2013).
- S. Iman, M. Aqil, M., Akhtar, Y. Sultana, A. Ali, Anal. Methods, 6, 282, (2014).
- M.A. Usman, M. Aminu, R.B. Oloyede, NJSR, 14, 41-46, (2015).
- A.S. Patil, J.J. Karanjavkar, K.S.K..,Khatik, I.S.K. Kathik, IJAPA, 5, 58- 60, (2015).
- M.S. Kumar, A.A. Smith, G.A. Vasagam, A.K. Muthu, R. Manavalan, IJPSR, 2,122-126, (2010).
- C.Danel, C. Barthélémy, D. Azarzar, H.Robert, J.P Bonte, P.Odou, C. Vaccher, J. Chromatogr. A, 1163, 228-236, (2007).
- F. Nagase, A. Asato, P. López, A. Gaona, M.I. Santoro, E. Kedor, M. Aurora-Prado, Curr. Anal. Chem.,10, 205-215, (2014).
- R. Dahiya, J. Patel, S.K. Sahu, Asian J. Chem., .29, 99-103, (2017).
- A.E. Zeinab, B. El-Zeany, M. El-Houssini, J.Pharm.Anal., 36, 975-981, (2005).
- S. Singh, D. Rajiv, J. Patel,S. Kumar Sahu, S. Kumar Gupta, Asian J. Chem., 29, 99-103, (2016).
- ICH Guidelines, Q2 (R1), Validation of analytical procedures: test and methodology, November, 2005.
- International Federation of Pharmaceutical Manufactures & Associations (IFPMA) Validation of analytical procedure. In: Proceedings of the International Conference on Harmonization (ICH ‘96), Methodology Q2(R1). International Federation of Pharmaceutical Manufactures& Associations (IFPMA), Geneva, 1996.
- FDA Reviewer Guidance Validation of Chromatographic Methods. Center for Drug Evaluation and Research (CDER). Analytical Methods Technical Committee of the Chemistry Manufacturing Controls Coordinating Committee (CMC CC) of the Center for Drug Evaluation and Research at the Food and Drug Administration, Rockville, 1994.ahiya, J. Patel, S.K. Sahu, Asian J. Chem., .29, 99-103, (2017).


