- Anilinomethylenemalonate,
- Room temperature synthesis,
- Quinoline,
- Methanolysis
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
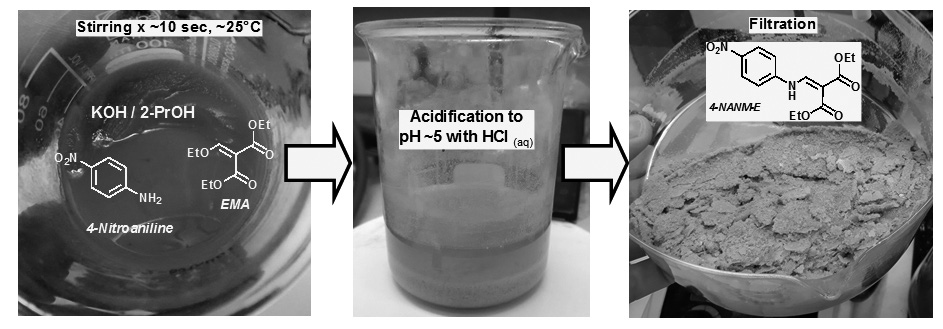
Diethyl 2-((4-nitroanilino)methylene)malonate [4-NANM-E] is an important molecule owing to its role of precursor in the multistage synthesis of several quinoline derivatives possessing biological activities such as antiviral, immunosuppressive, anticancer and photoprotector. This molecule is usually synthesized by a nucleophilic vinyl substitution (SNV) between 4-nitroaniline and diethylethoxymethylene malonate (EMA). Although several procedures are available to synthesize 4-NANM-E in liquid phase, more convenient method is necessary to synthesize in less reaction time and at room temperature. In this study, it is demonstrated that equimolar amounts of EMA and 4-nitroaniline dissolved in alcoholic KOH react within a few seconds at room temperature to produce 4-NANM-E which is purified by simple filtration after acidification with aqueous HCl and washing with alcohol. The reaction has the yield varying at the range 45-53% when it occurs in ethanol, 2-propanol, 2-butanol or 2-pentanol. Therefore, this synthesis method is an excellent alternative to produce 4-NANM-E on an industrial scale.
References
- M. Artico, A. Mai, G. Sbardella, S. Massa, C. Musiu, S. Lostia, F. Demontis, P. La Colla, Bioorganic Med. Chem. Lett. 9, 1651, (1999).
- M.V. Shul’gina, N.I. Fadeeva, T.N. Bol’shakova, I.B. Levshin, R.G. Glushkov, Pharm. Chem. J. 33, 343, (1999).
- S. Sarkar, P. Ghosh, A. Misra, S. Das, Synth. Commun. 45, 2386, (2015).
- T. Stärhfeldt, Patent US006172232B1, 2001.
- J.A. Tucker, V.A. Vaillancourt, J.W. Strohbach, K.R. Romines, M.E. Schnute, M.M. Cudahy, S. Thaisrivongs, S.R. Turner, Patent US006093732A, 2000.
- J.-F. He, L.-H. Yun, R.-F. Yang, Z.-Y. Xiao, J.-P. Cheng, W.-X. Zhou, Y.-X. Zhang, Bioorg. Med. Chem. Lett. 15, 2980, (2005).
- B. Lucero, C. Gomes, I. Frugulhetti, L. Faro, L. Alvarenga, M. De Souza, T. De Souza, V. Ferreira, Bioorganic Med. Chem. Lett.16, 1010, (2006).
- M.P. Moyer, F.H. Weber, J.L. Gross, J.W. Isaac, R. Fort, Bioorg. Med. Chem. Lett. 2, 1589, (1992).
- F. Boechat, C. Sacramento, A. Cunha, F. Sagrillo, C. Nogueira, N. Fintelman-Rodrigues, O. Santos-Filho, C. Riscado, L. Forezi, L. Faro, L. Brozeguini, I. Marques, V. Ferreira, T. Souza, M. De Souza, Bioorganic Med. Chem. 23, 7777, (2015).
- A.P. Kaplan, V. Gupta, J.W.F. Wasley, Patent US 20080306049A1, 2008.
- D. Yang, L. Arifhodzic, C.R. Ganellin, D.H. Jenkinson, Eur. J. Med. Chem. 63, 907, (2013).
- C. Oh, I. Yi, K.P. Park, J. Heterocycl. Chem. 31, 841, (1994).
- H. Agui, T. Mitani, M. Nakashita, T. Nakagome, J. Heterocyclic Chem. 8, 357, (1971).
- B. Riegel, G.R. Lappin, B.H. Adelson, R.I. Jackson, C.J. Albisetti, R.M. Dodson, R.H. Baker, J. Am. Chem. Soc. 68, 1264, (1946).
- G.F. Duffin,; J.D. Kendall, J. Chem. Soc. 893, (1948).
- D. Tarabová, V. Milata, J. Hanusek, Acta Chim. Slovaca. 6, 73, (2013).
- K.-W. Kim, H.-J. Lee, J.-I. Jo, T.-W. Kwon, Bull. Korean Chem. Soc. 31, 1155, (2010).
- C. Leonelli and P. Veronesi, In Production of Biofuels and Chemicals with Microwave, Z. Fang, R.L. Smith Jr., X. Qi, Eds. Springer Netherlands, Dordrecht, 2015; pp. 17–40.
- A. Gómez-Sanchez, E. Sempere, J. Bellanato, J. Chem. Soc. Perkin Trans. 2. 3, 561, (1981).
- D. M. Mulvey, R.J. Tull, L.M. Weinstock, Patent US3515745A, 1970.
- N. Katagiri, H. Akatsuka, T. Haneda, C. Kaneko, A. Sera, J. Org. Chem. 53, 5464, (1988).
- S. Antus, F. Boross, M. Nógrádi, Justus Liebigs Ann. Chem. 1, 107, (1978).
- I.A. Wolff, D.W. Olds, G.E. Hilbert, Synthesis (Stuttg). 9, 732, (1984).
- D. Kusdiana, S. Saka, Fuel. 80, 693, (2001).


