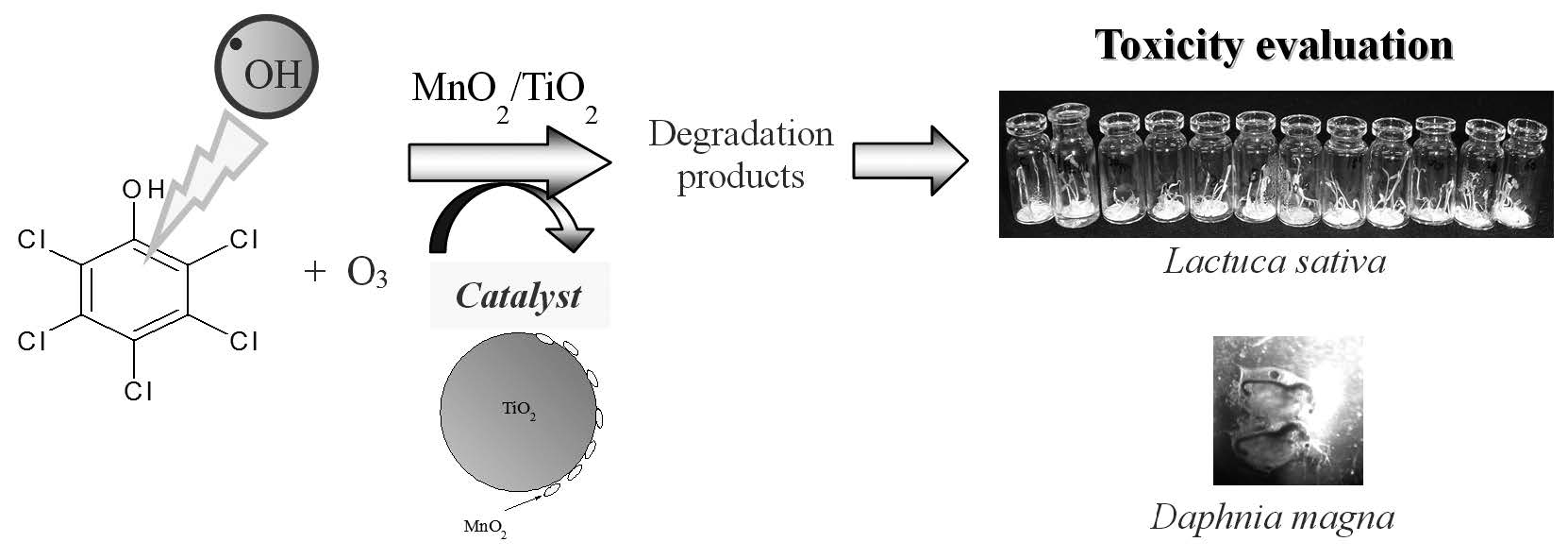
TOXICITY STUDIES DURING THE DEGRADATION OF PENTACHLOROPHENOL BY OZONATION IN THE PRESENCE OF MnO2/TiO2
- Pentachlorophenol (PCP),
- catalytic ozonation,
- MnO2/TiO2,
- toxicity assays,
- Lactuca sativa
- Panicum millaceum,
- Daphnia magna ...More
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Ozone is a strong oxidant used in the water treatment to remove organochloride compounds. Given that many processes of degradation generate chemical compounds that are more toxic than initial compounds, the development of optimized ozonation processes are required. In this study, pentachlorophenol (PCP) was used as a model of an organochlorine compound and the toxicity of its degradation products generated by both non-catalytic and catalytic ozonation processes were evaluated with a fresh-water Daphnia magna (ecotoxicity) and vegetables species Lactuca sativa and Panicum millaceum (Phytoxicity). The catalytic ozonation used MnO2/TiO2 as catalyst, which was characterized by X-ray diffraction analysis showing the presence of crystalline TiO2 phases, rutile and anatase. The specific BET surface area of MnO2/TiO2 was 43 m2/g. It was found that the use of ozone as an oxidant showed a first order degradation rate constant (kobs = 0.5 ± 0.1 min- 1). The uncatalyzed reaction showed several reaction intermediates like mono- and polychlorinated phenols, and quinones. The oxidation of these compounds led to low molecular weight organic acids. From these results, we proposed a pathway of PCP degradation using ozone. The catalyzed reaction showed a more potent effect in reducing the toxicity of the treated solution. Unlike the use of only ozone which does not decrease the toxicity. It was found that the treatment with catalytic ozonation decreases the toxicity of the solutions.
References
- Orton F., Lutz I., Kloas W., Routledge E.J., Environ Sci Technol., 43, 2144-2150, (2009).
- ATSDR. Agency for Toxic Substances and Disease Registry, Toxicological Profile for Pentachlorophenol. Department of Health and Human Services, Public Health Service. Atlanta, United States, 2001.
- Farré M.J., Franch M.I., Malato S., Ayllón J.A., Peral J., Doménech X., Chemosphere, 58, 1127-1133, (2005).
- U.S. E.P.A. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Pentachlorophenol.
- National Center for Environmental Assessment, Office of Research and Development, Washington, DC, 1999.
- Oturan M.A., Oturan N., Lahitte C., Trevin S., J Electroanal. Chem., 507, 96-102, (2001).
- EPA. Environmental Protection Agency. National Recommended Water Quality Criteria. Federal Register 57-60848, 1992.
- Miháltz P., Pál F., Siska J., Duchateau F., Cent Eur J Public. Health., 8, 12-13, (2000).
- Luthe C.E., Chemosphere, 32, 2409-2425, (1996).
- Xiao Xiao, Jianfang Hu, Ping’an Peng, Deyi Chen, Xinhui Bi. Environ. Pollut., 212, 464-471, (2016).
- Yoshida K., Nakanishia J., Chemosphere, 53, 427-436, (2003).
- Pohl H.R., McClure P., De Rosa C.T., Environ. Toxicol. Pharmacol., 18, 259-266, (2004).
- Von Gunten U., Water Res. 37, 1443-1467, (2003).
- Esplugas S., Bila D.M., Krause L.G.T., Dezotti M., J. Hazard. Mater., 149, 631–642, (2007).
- Dantas R.F., Contreras S., Sans C., Esplugas S., J. Hazard. Mater., 150, 790–794, (2008).
- Jardim W.F., Moraes S.G., Takiyama M.M.K., Water Res., 31, 1728-1732, (1997).
- Hong P.K.A., Zeng Y., Water Res., 36, 4243-4254, (2002).
- Li X., Cao X., Wu G., Temple T., Coulon F., Sui H., Chemosphere, 109, 71–76, (2014).
- Matilainen A., Sillanpää M., Chemosphere, 80, 351-365, (2010).
- Kasprzyk-Hordern B., Zió ek M., Nawrocki J., Appl. Catal. B: Environ., 46, 639-669, (2003).
- Chen K.C., Wang Y.H., Chem. Eng. Journal., 253, 84-92, (2014).
- Chen Y.-H., Hsieh D.-C., Shang N.-C., J. Hazard. Mater., 192, 1017– 1025, (2011).
- Kasprzyk-Hordern B., Raczyk-Stanislawiak U., Swietlik J., Nawrocki J., Appl. Catal. B: Environ., 62, 345–358, (2006).
- Rosal R., Gonzalo M.S., Rodriguez A., Garcia-Calvo E., J. Hazard. Mater., 169, 411–418, (2009).
- Quispe C., Villaseñor G., Pecchi P., Reyes P., J. Chil. Chem. Soc., 51, 1049–1052, (2006).
- Villaseñor J., Reyes P., Pecchi G., Catal. Today, 76, 121-31, (2002).
- Hunter R.J.J. Zeta Potential in Colloid Science, Principles and Applications, Academic Press, New York, 1981.
- Villaseñor J., Mansilla H.D., J. Photochem. Photobiol., 93, 205-209, (1996).
- APHA. American Public Health Association, Standard Methods for the Examination of Water and Wastewater. In Greenberg, A. E., L. S. Clesceri & A. D. Eaton (eds), Washington, 1992.
- Cutillo F., D’Abrosca B., DellaGreca M., Di Marino C., Golino A., Previtera L., Zarrelli A., Phytochem., 64, 1381-1387, (2003).
- Cazar M.E., Schmeda-Hirschmann G., Astudillo L., World J. Microbiol. Biotechnol., 21, 1067-1075, (2005).
- NCh2083. Norma Chilena Oficial Chile. Agua-Bioensayo de toxicidad aguda mediante la determinación de la inhibición de la movilidad de Daphnia magna o Daphnia pulex (Crustacea, Cladocera), 1999.
- Pérez M., Torrades F., Domènech X., Peral J. J. Chem. Technol. Biotechnol., 77, 891-97, (2002).
- Chang C.Y., Chen Y.H., Li H., Chiu C.Y., Yu Y.H., Chiang P.C., Ku Y., Chen J.N., J. Environ. Eng. ASCE., 127, 908-915, (2001).
- Staehelin S., Hoigne J., Environ. Sci. Technol., 16, 676–681, (1982).
- Legrini O., Oliveros E., Braun A.M. Chem Rev., 93, 671-698, (1993).
- Anipsitakis G.P., Dionysiou D.D., Gonzalez M.A. Environ. Sci. Technol., 40, 1000-1007, (2006).
- Pecchi G., Reyes P., Sanhueza P., Villaseñor J., Chemosphere, 43, 141- 146, (2001).
- Fernández-Alba A., Hernando D., Agüera A., Cáceres J., Malato S., Water Res., 36, 4255-4262, (2002).
- Bozzi A., Dhananjeyan M., Guanaquillo I., Parra S., Pulgarin C., J. Photochem. Photobiol. A: Chem., 162, 179–185, (2004).
- Santos A., Yustoa P., Quintanilla A., Garcia-Ochoa F., Casas J.A., Rodríguez J., Environ. Sci. Technol., 38, 133-138, (2004).
- Azevedo E., Radler de Aquino F., Dezotti M., Appl. Catal. B: Environ., 54, 165-173, (2004).
- Silva J., Iannacone A., Cifuentes L., Troncoso E., Bay-Schmith E., Larrain A., Ecotoxicol. Environ. Restor., 4, 10-17, (2001).


