Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
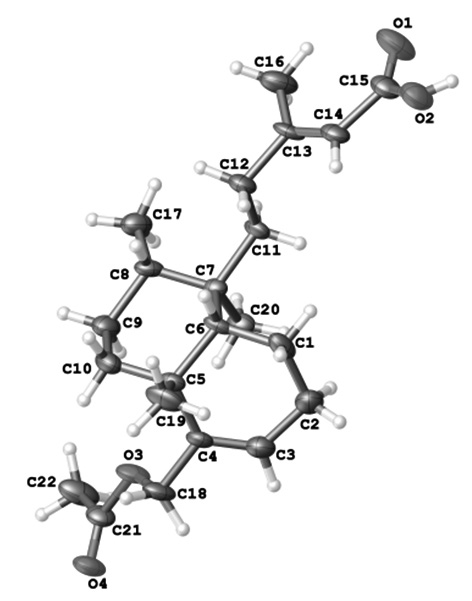
In this paper we report the absolute configuration which has been determined from the refinement of the Flack parameter, x = 0.05(5), which indicate that the correct configuration had been assigned against 1353 (99%) CuKα Bijvoet pairs. On this basis the absolute configuration was assigned as C5R, C8S, C9R and C10S. The structure of 18-acetoxy-cis-cleroda-3,13E-dien-15-oic acid consists of a clerodane skeleton and the corresponding methyl groups are α-oriented (C8, C9) while C5 is β-oriented. The acidic lateral chain is β-oriented and the double bond between C13 and C14 has E isomeric. The ciclohexene, cyclohexane rings are cis fused, and in an sofa and chair conformation respectively. In the crystal the molecules are linked by one intermolecular OH⋅⋅⋅O hydrogen bond forming 1D-dimensional chain with distance donor-acceptor of 2.060(6)Å with graph-set notation C1 1 (15).
References
- M. Montes, T. Wilkomirsky. Medicina Tradicional Chilena, Universidad de Concepción, Concepción, p. 193, (1985).
- A. Urzua, R. Torres, M. Muñoz, Y. Palacios. Journal of Ethnopharmacology, 45, 71, (1995).
- H.Vogel, M. González, F. Faini, I. Razmilic, J. Rodriguez, J.S. Martin and F. Urbina Journal of Ethnopharmacology 97, 97-100, (2005).
- F. Faini, R. Torres, J.M. Rodilla, C. Labbe,C. Delporte and F. Jaña. J. Braz. Chem. Soc. 22, 2344, (2011).
- G. Schmeda-Hirschmann, C. Quispe and B. González. Molecules. 20, 913, (2015).
- G.E. Wickens. Vegetation and ethnobotany of the Atacama Desert and adjacent Andes northern Chile. Opera Bot., 121, 291, (1993).
- N. Monterrey. Hierbas Medicinales Andinas 2a Región. Ministerio de Educación, Antofagasta, p. 35, (1996).
- A. Urzua, F. Jara, E. Tojo, M. Wilkens, L. Mendoza, M.M. Rezende. Journal of Ethnopharmacology 297, 103, (2006).
- G. Morales, P. Sierra, L.A. Loyola, J. Bórquez. Phytochemistry. 55, 863, (2000).
- J. Bórquez, A. Mancilla, S. Pedreros, L.A. Loyola, G. Morales, O. Wittke, I. Brito. Bol. Soc. Chil. Quím., 40, 157, (1995).
- E. Tojo, M.E. Rial, A. Urzua, L. Mendoza. Phytochemistry, 52, 1531, (1999).
- J. Bórquez, A. Ardiles, L.A. Loyola, L.M. Peña-Rodriguez, G.M. Molina- Salinas, J. Vallejos, G. Isidro, I.G. Collado, M.J. Simirgiotis. Molecules, 19, 3898, (2014).
- J. Bórquez, C. Echiburú-Chau, P. Winterhalter, J. Vallejos, G. Jerz, M.J. Simirgiotis.. Journal of Science of Food and Agriculture, 96, 2832, (2016).
- J. Borquez, E.J. Kennelly and M.J. Simirgiotis. Food Research International 52, 288, (2013).
- I. Brito, J. Borquez, M. Simirgiotis, A. Cardenas and M. Lopez-Rodriguez. Acta Crystallographica Section E-Structure Reports Online. E68, o32. (2012).
- I. Brito, J. Bórquez, M.J. Simirgiotis, M. Neves-Vieira, G. Jerz, P. Winterhalter, M. Bolte, A. Cárdenas. Zeitschrift für Kristallographie- New Crystal Structures, 229, 399, (2014).
- I. Brito, M.J. Simirgiotis, R. Muñoz, J. Benites, L. Pasten, J. Bórquez, A Cárdenas. Zeitschrift für Kristallographie-New Crystal Structures, 232, 13, (2017).
- Simirgiotis. Saudi Pharmaceutical Journal 25, 1032, (2017).
- H.D. Flack. Acta Cryst. A39, 876, (1983).
- G. M. Sheldrick, Acta Cryst. 112, A64, (2008).
- O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H. Puschmann, J. Appl. Cryst., 42, 339, (2009).
- D.Cremer, J.A. Pople. J.Am.Chem.Soc. 97, 1354, (1975).
- J. Bernstein, R.E. Davis, L. Shimoni, N.-L. Chang. Angew. Chem. Int. Ed. Engl. 34, 1555, (1995).


