- Chromium,
- alginate,
- nanoparticles,
- adsorption,
- emulsion cross linking
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
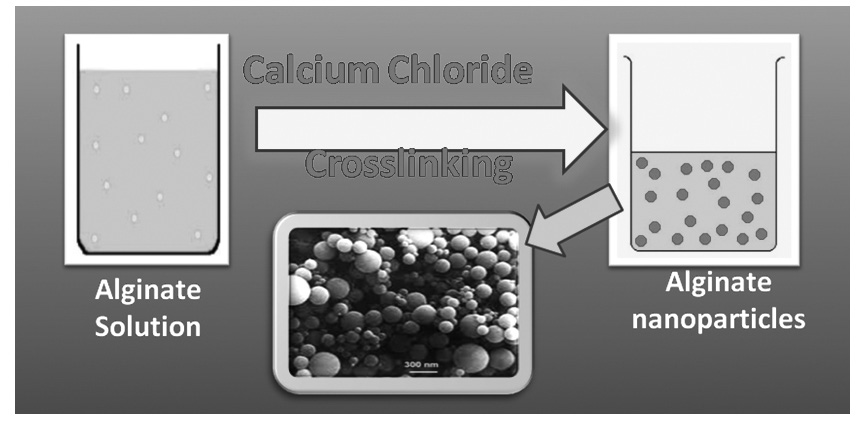
The objectives of the present work include synthesis of nanoparticles of calcium alginate by emulsion crosslinking method, their characterization by techniques such as FTIR, scanning electron microscopy (SEM), XRD, particle size and surface charge analysis, and study of removal of chromium ions from synthetic aqueous solutions. In order to study the effect of various factors like adsorbent dose, initial metal ion concentration, pH and temperature on the adsorption of chromium ions from water, a series of batch sorption experiments were conducted. The obtained results were analysed by Langmuir and Freundlich isotherm models and it was found that the adsorption of chromium more precisely follows Langmuir absorption isotherm. The metal ion removal capacity of the nanoparticles was found to depend on the chemical composition of the adsorbent, concentration of the metal ion solution, pH and temperature of the medium and speed of the suspension from wastewater before disposal.
References
- J.N.Moore, S.N. Lumoa, Environ. Sci. Technol. 2, 24 (1990).
- H.Akcay, A.Oguz, C.Karapire, Water Res. 813, 37(4) (2003).
- P. B Tchounwou, C. G Yedjou, A.K Patlolla and D.J Sutton. HHS Public Access, 101:133-164.(2012).
- S.R Mousavi, M balali-Mood, B. Riahi-Zanjani, H. Yousefzadeh, M sadeghi, Int.J. Occup. Env. Med. 4, 2 (2013).
- A. Chehregani and B. Malayeri , Int.J.Agri..Biology, 1560, 462(2007).
- D.Balamurugan, C.Udayasooriyan, B. Kamaldevi, Int.J.Environ.Sci.5(3), 522 (2014).
- P. Miretzky, A.Saralegui, A. F Cirelli, Chemosphere 57, 997 (2004).
- V. I. Slaveykova, K. J. Wilkinson, Environ. Sci. Technol. 36, 969 (2002).
- E. J. J. Kalis, L. P. Weng, F. Dousma , E. J. M. Temminghoff, W. H. Van Riemsdijk., Environ. Sci. Technol.40, 955 (2006).
- H.Sigel (Ed.), Dekker, New York, 24,(1988).
- A. Dashti, M. Soodi, N. Amani, Environ. Toxicol.31 (3), 269(2016).
- A. Dasht, M. Soodi, N. Amani, Pathobio.Res. 18, 55(2015).
- A.Plech, T. Klimkiewicz, H. Jakrzewska, J. Env. Studies, 9(4) 301(2000).
- B.A. Adelekan and K.D. Abegunde, Int. J. Phy. Sci. 6, 1045(2011).
- R.A Goyer. Mc Graw-Hill Publisher,811 (2001).
- P.Saranraj and D.Sujitha International J. Microbiol. Res. 4 (3), 305 (2013).
- M Hema, K Srinivasan, Ind. J. Eng. Materl.17, 373 (2010).
- R. Taman, M. E. Ossman, M. S. Mansour, H. A. Farag, J. Adv. Chem. Eng. 5, 125(2015).
- J.N.Bhakta, P. B. Majumdar, and Y. Munekage, Int.J.Chem.Eng. Vol 201 (2014).
- V.Baheti, V.Vellora T. Padil, J. M Cernik, R Mishra. J. Fiber Bioeng. Informatics 6, 175(2013).
- K.A. Adegoke, O. S. Bello, Wat.Res.Ind., 12, 8 (2015).
- S.K Halnor and M. Ubale, J. Appl. Chem.., 2(3), 475 (2013).
- I. Ahmad, S. Zafer, F. Ahmad . J Appl Sci Environ. 9 (1),123 (2005).
- G..Donmez. Z. Aksu . Biochemistry, 38(5), 751(2002).
- Z. Hu ,L. Lei,Y. Li. And Y. Ni Sep.Purif.Technol. 31(1), 13 (2003).
- M. Lotfi And N. Adhoum , Sep.Purif.Technol. 26(2-3), 137(2002).
- M. M. Rao, A. Ramesh, G.P.C Rao, K. Seshaiah, J. Hazard Mater, 129, 123 (2006).
- M.A.Barakat, Arabian J.Chem. 4, 361 (2011).
- A.Padilla, E.L Tavani, Desalination, 129(1-3), 219-226(1999).
- M.A Barakat, Jamshed Rashid, M.A Alghamdi., , Seventeenth International water technology conference, IWTC17, Istanbul, 5-7 November 2013.
- L.Wu, L. Liao, G. Lv, F. Qin, Y. He, X. Wang, J. Haz. Materials 254,277(2013).
- A.K Bajpai, R Sachdeva. J Appl Polym Sci. 78, 1656 (2000).
- S.Gang and S. Weixing. Ind. Eng.Chem.Res., 37(4),1324(1998).
- A.K Bajpai , N. Vishwakarma Colloids Surface A - Physicochem Eng Aspects ; 220:117(2003).
- A K Bajpai, L. Rai.,Ind.J.Chem.Technol. 17, 17 ( 2010).
- M.Doula, A.N Loam,A. Dimirkou. Commun Soil Sci Plant Anal. 26:1535. (1995).


