CATALASE, CATECHOLASE AND PHENOXAZINONE SYNTHASE-LIKE ACTIVITIES OF HOMODINUCLEAR Co(II), Ni(II), Cu(II) and Zn(II) COMPLEXES INCLUDING OXIME GROUP
- Homodinuclear metal complexes,
- oxidoreductases,
- catalase,
- catecholase,
- phenoxazinone synthase
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
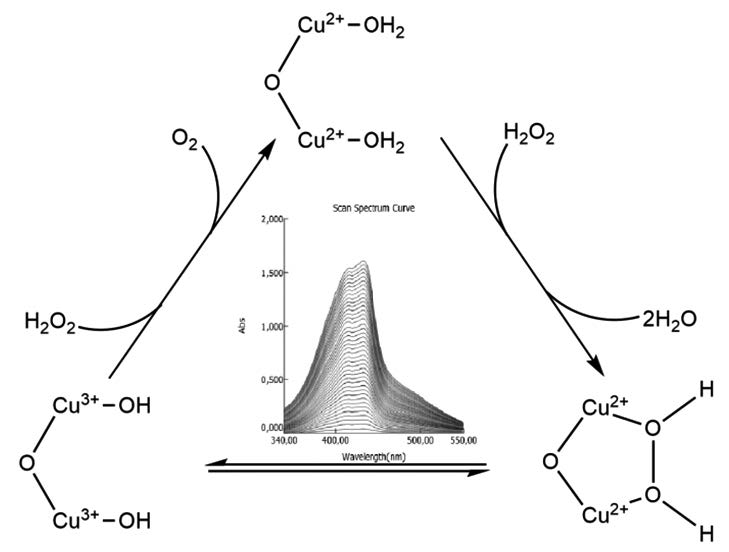
Homodinuclear (1) Co(II), (2) Ni(II), (3) Cu(II) and (4) Zn(II) metal complexes containing oxime group were tested for their catalase, catecholase and phenoxazinone synthase-like activity by volumetric and spectrophotometric procedures. Catalase-like activity of the complexes was studied by measuring the evolved dioxygen resulted from the disproportionation reaction of potentially harmful hydrogen peroxide. Catecholase and phenoxazinone synthase-like enzyme activities were followed by the increase in absorbance at 400 and 433 nm resulted from the oxidation reaction of 3,5-di-tert-butylcatechol to 3,5-di-tert-butylquinone and 2-aminophenol to 2-aminophenoxazine-3-one, respectively. Among the studied homodinuclear complexes, complex (3) showed the highest catalytic efficiency for three enzymes tested. Catalytic efficiency of the complexes was found 3>1>4=2 for catalase-like, 3>1>2>4 for catecholase-like and 3>1>4>2 for phenoxazinone synthase-like activity. Relatively higher catalytic activity of the Cu(II) complex is thought to be related to the lower redox potential of Cu(II) ion and better proximity of the chosen substrates with the complex (3).
References
- Y. Lu, J. S. Valentine, Curr. Opin. Struct. Biol. 7(4), 495, (1997).
- S. N. Raja, H. Y. Shrivastava, B. U. Nair, Ind. J. Chem, 50A, 531, (2011).
- R. K. Sharma, V. Krishnan, Bioinorganic Chemistry, NSDL, India, 2007.
- E. M. Peck, B. D. Smith, Synthetic Receptors for Biomolecules: Design Principles and Applications, RSC Publications, 2015.
- Z. Dong, Q. Luoa, J. Liu, Artificial enzymes based on supramolecular scaffolds, Chem. Soc. Rev.41, 7890, (2012).
- J. Paschke, M. Kirsch, H.-G. Korth, H. Groot, R. Sustmann, J. Am. Chem. Soc. 123, 11099, (2001).
- F. Yu, V. M. Cangelosi, M. L. Zastrow, M. Tegoni, J. S. Plegaria, A. G. Tebo, C. S. Mocny, L. Ruckthong, H. Qayyum, V. L. Pecoraro, Protein Design: Toward Functional Metalloenzymes, Chem. Rev. 114, 3495, (2014).
- B. Dede, F. Karipcin, M. Cengiz, J. Hazard. Mater. 163, 1148, (2009).
- A. Chakravorty, Coordin. Chem. Rev. 13, 1, (1974).
- P. Mitchell, Science, 206, 1148, (1979).
- M. C. M. Laranjeira, R.A. Marusak, A.G. Lappin, Inorg. Chim. Acta. 300, 186, (2000).
- J. R. Dilworth, S. Parrott, Chem. Soc. Rev. 27, 43, (1998).
- S. S. Jurisson, J. D. Lydon, Chem. Rev. 99, 2205, (1999).
- M. Bakir, J. A. M. McKenzie, J. Chem. Soc. Dalton Trans. 3571, (1997).
- R. Ruiz, F. Lloret, M. Julve, J. Faus, M. C. Munoz, X. Solans, Inorg. Chim. Acta, 213, 268, (1993).
- N. Sengottuvelan, J. Manonmani, M. Kandaswamy, Polyhedron, 21, 2767, (2002).
- J. Kaizer, R. Csonka, G. Speier, M. Giorgi, M. Reglier, J. Mol. Catal. A: Chem. 236, 12, (2005).
- V. Balzani, A. Juris, M. Venturi, S. Campagna, S. Serroni, Chem. Rev. 96, 759, (1996).
- F. Karipcin, B. Dede, M. Cengiz, Russ. J. Inorg. Chem. 55, 530, (2010).
- H. Aebi, Method. Enzymol. 105, 121, (1984).
- M. U. Triller, D. Pursche, W. Y. Hsieh, V. L. Pecoraro, A. Rompel, B. Krebs, Inorg. Chem. 42, 6274, (2003).
- J. Kaizer, G. Baráth, R. Csonka, G. Speier, L. Korecz, A. Rockenbauer, L. Párkányi, J. Inorg. Biochem. 102, 773, (2008).
- J, Reim, B. Krebs, J. Chem. Soc. Dalton. Trans. 3793, (1997).
- J. Gao, A. E. Martell, J. H. Reibenspies, Inorg. Chim. Acta. 346, 32, (2003).
- B. M. Pires, D. M. Silva, L. C. Visentin, B. L. Rodrigues, N. M. F. Carvalho, R. B. Faria, PLoS. ONE 10, 1, (2015).
- M. Shank, V. Barynin, G. C. Dismukes, Biochemistry-US, 33, 15433 (1994).
- Z-F. Chen, Z-R. Liao, D-F. Li, W-K. Li; X-G. Meng J. Inorg. Biochem, 98, 1315, (2004).
- A. Neves, A. J. Bortoluzzi, R. Jovito, R. A. Peralta, B. de Souza, B. Szpoganicz, A. C. Joussef, H. Terenzi, P. C. Severino, F. L. Fischer, G. Schenk, M. J. Riley, S. J. Smith, L R. Gahan, J. Braz. Chem. Soc. 21, 1201, (2010).
- J. P. Chyn, F. L. Urbach, Inorg. Chim. Acta. 189, 157, (1991).
- I. C. Szigyarto, T. M. Simandi, L. I. Simandi, L. Korecz, N. Nagy, J. Mol. Catal. A: Chem. 251, 270, (2006).
- M. Mitra, R. Ghosh, Indian J. Chem. A. 55, 681, (2016).
- M. Mitra, T. Kundu, G. Kaur, G. Sharma, A. R. Choudhury, Y. Singhd, R Ghosh, Roy. Soc. Ch. Adv. 6, 58831, (2016).
- L. I. Simándi, T. M. Barna, L. Korecz, A. Rockenbauer, Tetrahedron Lett. 34, 717, (1993).
- A. Panja, M. Shyamal, A. Saha, T. K. Mandal, Dalton Trans. 43, 5443, (2014).
- T. Horváth, J. Kaizer, G. Speier, J. Mol. Catal. A: Chem. 215, 9, (2004).


