COEFFICIENT PARTITION PREDICTION OF SATURATED MONOCARBOXYLIC ACIDS USING THE MOLECULAR DESCRIPTORS
- Octanol-Water Coefficient Partition,
- MLR Method,
- Saturated Monocarboxilic Acid
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
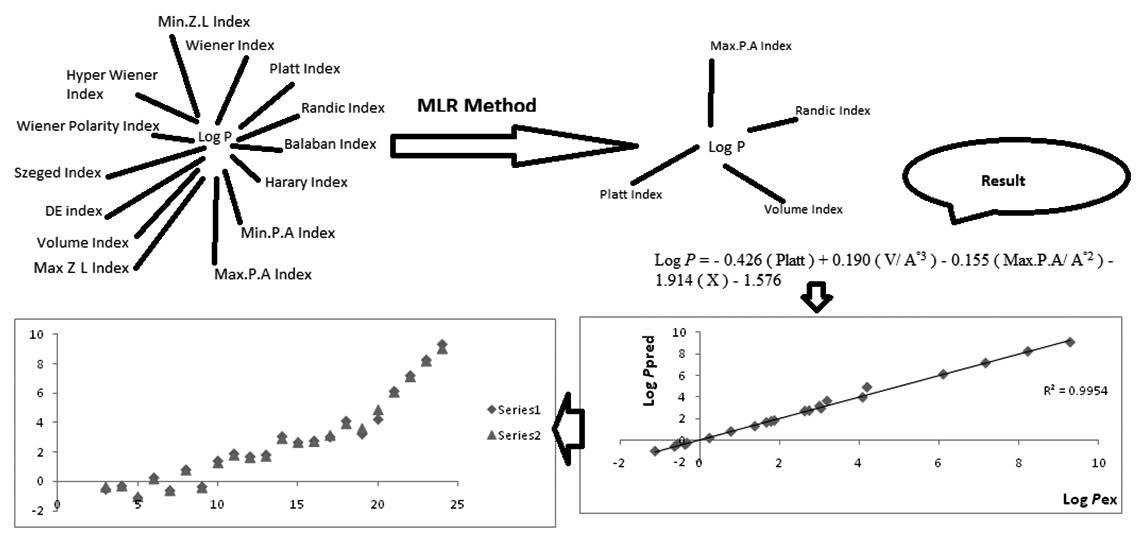
Carboxylic acids have clearly been absent from the quantitative structure-property relationship literature. The studies of the quantitative structure–property relationships (QSPR) involve various chemometric methods in which the physico-chemical behavior of a compound is correlated with its structure represented by the structural indices. For example, QSPR methods are applied for the prediction of octanol-water partition coefficient of an organic compound. In this study, the relationship between the octanol/water coefficient partition and molecular descriptors was investigated. Also, the multiple linear-regression method based on QSPR methodology was applied to predict the Log P of saturated mono-carboxylic acids C1-C22. On the other hand, the relation [ Log P = - 0.426 ( Platt ) + 0.190 ( V/ A°3 ) - 0.155 ( Max.P.A/ A°2 ) - 1.914 ( X ) - 1.576 ; N = 22, R2 = 0.995 , F = 917.005, DW=1.391] was generated for selected mono-carboxylic acids. The results of study indicated that the Platt, Randic, Volume and Maximum-Projection-Area descriptors have an important role in predicting the octanol/water coefficient partition of saturated monocarboxylic acids (C1- C22).
References
- W. Riemenschneider, Carboxylic Acids, Aliphatic, Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH, 2002.
- J. March, Advanced organic chemistry—reactions, mechanisms and structure. 4th edn, Wiley Interscience, New York, 8, 1992.
- J. R. Seward, T. W. Schultz, QSAR analyses of the toxicity of aliphatic carboxylic acids and salts to Tetrahymena pyriformis. SAR QSAR Environ Res. 10, 557, (1999).
- S. P. Torres, J. Sales, M. Rosés, C. Ràfols, E. Bosch , Journal of Chromatography A., 1217 (18), 3026, (2010).
- R. Smith and C. Tanford, Proc. Nat. Acad. Sci. USA, 70, (2), 289, (1973).
- F. Spafiu, A. Mischie, P. Ionita, A. Beteringhe, T. Constantinescu and A. T. Balabanb,. Arkivoc, General Papers, (x) 174, (2009).
- J. Devillers, A. T. Balaban, Topological indices and related descriptors in QSAR and QSPR. Gordon and Breach Science, Netherlands, 1999.
- E. Mohammadinasab, and M. Goodarzi, J. Fullerenes, Nanotubes, and Carbon Nanostructures., 19, 550, (2011).
- M. Goodarzi, E. Mohammadinasab, Fullerenes, Nanotubes and Carbon Nanostructures. 21, 2 ,102 (2013).
- M. Zanoozi and Z. Bayat, Pelagia Research Library, Der Chemica Sinica. 2(6), 288. (2011).
- Z. Bayat, J. Movaffagh, Russian Journal of Physical Chemistry A. 84, (13), 2293, (2010).
- G.W. Snedecor, W. G. Cochran, Statistical methods, Oxford and IBH, New Delhi, 1967.
- S. Wold, M. Sjöström, L. Eriksson. Chemometrics and Intelligent Laboratory Systems, 58, 109, (2001).
- A. Agresti, An introduction to categorical data analysis, Wiley, Hoboken, 1996.
- J. G. Topliss, R. J. Costello, J. Med Chem. 15, 1066, (1972).
- I. Gutman and O.E. Polansky, Mathematical Concepts in Organic Chemistry, Springer-Verlag, Berlin, 1986.
- R. Todeschini, V. Consonni, Handbook of Molecular Descriptors, Methods and Principles in Medicinal Chemistry, WILEY-VCH Verlag GmbH, 2008.
- I. Gutman, B. Furtula(eds), novel molecular structure descriptors-theory and applications I and II, University of Kragujevac and Faculty of Science Kragujevac, 2010.
- J. Sangster, Octanol-Water Partition Coefficients of Simple Organic Compounds, J. Phys. Chem. Ref. Data. 18, 3, (1989).
- H. Wiener, J. Am. Chem. Soc. 69, 17, (1947).
- G. Cash, S. Klavzar, Marko Petkovsek, J. Chem. Inf. Comput. Sci. 42, 571, (2002).
- X. Li, Y. Shi, MATCH Communications in Mathematical and in Computer Chemistry. 59 (1) 127, (2008).
- M. Randic, MATCH Commun. Math. Comput. Chem. 7, 5, (1979).
- B. Liu, I. Gutman, MATCH Communications in Mathematical and in Computer Chemistry., 58 (1), 147, (2007).
- M. Randic, J. Am. Chem. 97(23), 6609, (1975).
- A. T. Balaban and T. S. Balaban, Math. Chem. 8, 383, (1991).
- K. C. Das, B. Zhou and N. TrinajstiQ, J. Math. Chem. 1369, (2009).
- I. Gutman, Graph Theory Notes of New York. 27, 9, 1994.
- P. V. Khadikar, N. Deshpande, P. P. Dobrynin, J. Chem. Inf. Compt. Sci.35, 547 (1995).
- Web search engine developed by ChemAxon; software available at, http:// WWW. Chemicalize. Org.


