DETERMINATION OF HYDROXYZINE BY DIFFERENTIAL PULSE ANODIC VOLTAMMETRY USING CARBON PASTE ELECTRODE
- Hydroxyzine dihydrochloride,
- differential pulse anoding voltammetry,
- carbon paste electrodes,
- pharmaceutical dosage form,
- human urine
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
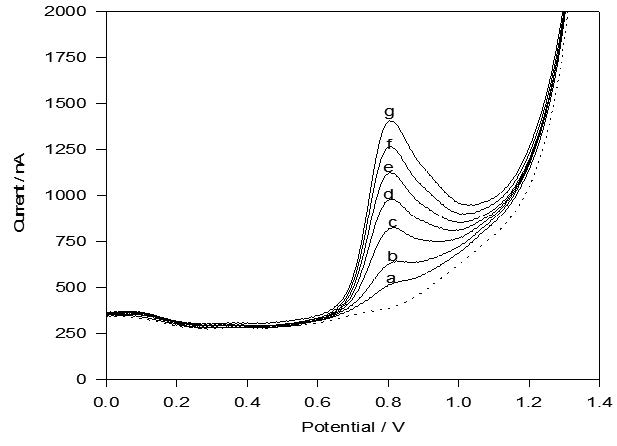
The electrochemical oxidation of hydroxyzine dihydrochloride at carbon paste electrode has been studied in 0.04 M Britton-Robinson buffer pH 6.2 using cyclic and differential pulse voltammetry. The oxidation process has been shown to irreversible and diffusion controlled with adsorption characterstics. Based on these studies, simple, rapid, and sensitive differential pulse anodic voltammetric method has been developed for the determination of the drug over the concentration range 0.45-5.36 μg/ml, with detection and quantification limits of 0.27 and 0.90 μg/ml hydroxyzine dihydrochloride, respectively. The proposed method was successfully used for quantification of hydroxyzine dihydrochloride in Atarax tablets and spiked human urine.
References
- J. Tsau, and N. DeAngelis, Analytical Profiles of Drug Substances1978; vol. 7, 321.
- G. N. Menon, B. J. Norris, J. Pharm. Sci., 70, 697, (1981).
- S. E. Roberts, M. F. Delaney, J. Chromatogr., 242, 364, (1982).
- A. N. Papas, S. M. Marchese, F. Delaney, LC Magazine, 2, 120, (1984).
- D. Boberic-Borojevic, D. Radulovic, D. Ivanovic, P. Ristic, J. Pharm. Biomed. Anal., 21, 15, (1999).
- S. F. Hammad, M. M. Mabrouk, A. Habib, H. El Fatatry, N. Kishikawa, K. Nakashima, N. Kuroda, Biomed. Chromatogr., 21, 1030, (2007).
- Z. Neng, L. Yi-Zeng, C. Ben-Mei, W. Ping, C. Xian, L. Feng-Ping, Chromatographia, 66, 481 (2007).
- Z. Bikui, C. Benmei, Z. Yungui, L. Huande, M. Ning, L. Wu, F. Sheng, Yaowu Fenxi Zazhi, 28, 516, (2008).
- A. F. B. Marcos, G. B. N. Luiz, C. P. Hudson, G. R. Fonseca, M. Gabriela, P. V. Urias, R. B. R. Nadia, N. G. Livia, O. F. Anderson, Latin American Journal of Pharmacy, 30, 1798 (2011).
- R. Alswayeh, S. N. Alvi, M. M. Hammami, World journal of Pharmacy and Pharmaceutical Sciences, 4, 127 (2015).
- N. Sher, F. A. Siddiqui, N. Fatima, S. Perveen, N. Shafi, J. Liq. Chromatogr. & Rel Techn., 38, 911 (2015).
- P. Kintz, B. Godelar, P. Mangin, Forensic Sci. Int., 48, 139, (1990).
- H. Ackermann, F. Kretzschmann, S. Kruger, B. Lexow, Nahrung, 21, 603, (1977).
- R. T. Sane, C. H. Thombare, P. G. Anaokar, A. D. Pandit, Indian Drugs, 18, 295, (1981).
- N. Rajendraprasad, K. Basavaiah, K. B. Vinay, H. D. Revanasid dappa, J. Mex. Chem. Soc., 54, 233, (2010).
- N. Rajendraprasad, K. Basavaiah, K. B. Vinay, J. Serb. Chem. Soc., 76, 1551, (2011).
- A. Mumtaz, S. Hussain, M. Yasir, Pak. J. Pharm. Sci., 27, 1157, (2014).
- Y. H. Ho, H. L. Wu, S. M. Wu, S. H. Chen, H. S. Kou, Anal. Bioanal. Chem., 376, 859, (2003).
- A. Bouklouze, M. Elbourekraoui, Y. Cherrah, M. Hassar, J-M. Kauffmann, Electroanalysis, 14, 1369, (2002).
- M. Javanbakht, S. E. Fard, A. Mohammadi, M. Abdouss, M. R. Ganjali, P. Norouzi, L. Safaraliee, Anal. Chimica Acta, 612, 65, (2008).
- A. A. Wassel, Anal. Bioanal. Electrochem., 4, 17, (2012).
- R. Mikulski, B. Dembinski, Anal. Chimica Acta, 272, 233, (1993).
- A. M. Beltagi, O. M. Abdallah, M. M. Ghoneim, Talanta, 74, 851, (2008).
- F. Huang, Y. Peng, G. Jin, S. Zhang, J. Kong, Sensors, 8, 1879, (2008).
- A. Elyacoubi, S. I. M. Zayed, B. Blankert, J-M., Kauffmann, Electroanalysis, 18, 345, (2006).
- M. Swartz, and I. S. Krull, Analytical Method Development and Validation, Marcel Dekker, Inc., 1997; 61.
- The United States Pharmacopeia, 38, The National Formulary 33, United States Pharmacopeial Convention, 2015; 3815.
- J. C. Miller, and J. N. Miller, Statistics for Analytical Chemistry, 3rd ed., Ellis Horwood, Chichester, 1993; 53.
- S. Kacew, Drug Toxicity & Metabolism in Pediatrics, US CRC Press Inc., 1990; 257.


