SYNTHESIS AND CHARACTERIZATION OF AN AMIDOXIME COMPOUND WITH BENZIMIDAZOLE MOIETY AND PH SENSING BEHAVIOR UNDER BASIC CONDITION IN METHANOL AND DMSO
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
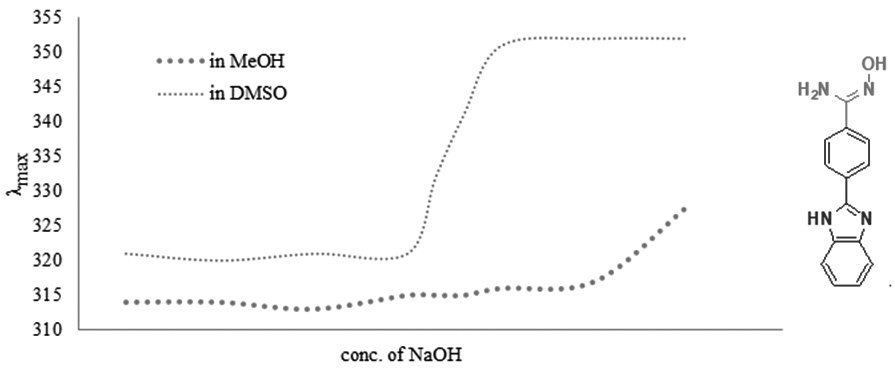
An amidoxime containing 2-phenyl-1H-benzimidazole derivative, 4-(1H-Benzoimidazol-2-yl)-N-hydroxy-benzamidine, was synthesized by conversion of nitrile group of previously synthesized 2-(4-cyanophenyl)-1H-benzimidazole by treatment with hydroxylamine under alkaline solution. Characterization of both compounds was carried out by using UV-Vis, FT-IR, 1H NMR and 13C NMR, and mass spectroscopic technique. Its photophysical behavior was compared in methanol and DMSO under basic condition.
References
- A. B. Goel, V. D. Gupta, J. Organomet. Chem. 72, 171, (1974).
- (a) H. Egawa, M.Nakayama, T. Nonaka, E. Sugihara, J. Appl. Polym. Sci. 33, 1993, (1987), (b) H. Egawa, T. Nonaka, S. Abe, M. Nakayama, J.Appl. Polym. Sci. 45, 837, (1992), (c) N. Kabay, H. Egawa, J. Appl. Polym. Sci. 51, 381, (1994), (d) N. Kabay, T. Hayashi, A. Jyo, H. Egawa, J. Appl. Polym.Sci. 54, 333, (1994), (e) Y. Zhaoa, J. Lib, L. Zhaod, S. Zhanga, Y. Huanga, X. Wua, X. Wanga, Chem. Eng. J. 235, 275, (2014).
- H. Egawa, N. Kabay, T. Shuto, A. Jyo, J. Appl. Polym.Sci. 46, 129, (1992).
- H. Kubota and Y. Shigehisa, J. Appl. Polym. Sci. 56, 147, (1995).
- A. Nilchia, A. A. Babaloub, R. Rafieeb, H. Sid Kalala, React. Funct. Polym. 68, 1663, (2008).
- Y. Wang, X. Ma, Y. Li, , X. Li, L. Yang, L. Ji, Y. He, Chem. Eng. J. 209, 394, (2012).
- (a) R. Qua, Y. Zhanga, W. Qub, C. Suna, J. Chena, Y. Pinga, H. Chena, Y. Niua, Chem. Eng. J. 219, 51, (2013), (b) J. Chen, R. Qu, Y. Zhang, C. Sun, C. Wang, C. Ji, P. Yin, H. Chen, Y. Niu, Chem. Eng. J. 209, 235, (2012).
- B. Gao, , Y. Gao, Y. Li, Chem. Eng. J. 158, 542, (2010).
- M. R. Lutfor, S. Silong, W. M. Zin, M.Z. Ab Rahman, M. Ahmad, J. Haron, Euro. Polym. J. 36, 2105, (2000).
- I. N. Voloschik, M. L. Litvina, B. A. Rudenko, J. Chromatogr. A 671, 51, (1994).
- P. A. Riveros, Hydrometallurgy 25, 1, (1990).
- J. L. Bernal, M. J. del Nozal, L. Debán, J. L. del Valle, V. Cerdá, J. M. Estela, J. Thermal Anal. 31, 931, (1986).
- J. L. Bernal, M. Pedrero, V. Cerdá, J. Cantallops, Thermochim. Acta. 98, 19, (1986).
- J. L. Bernal, M. J. Del Nozal, L. Debán, B. Nuñez, V. Cerdá, J. M. Estela, Thermochim. Acta. 103, 259, (1986).
- R. Jara, V. Cerdá, Thermochimic. Acta. 142, 135, (1989).
- M. Guillemet, A. Robert, M. Baudy-Floc’h, Tetrahedron Lett., 36, 547 (1995).
- C. A. Ramsden, H. L. Rose, J. Chem. Soc., Perkin Trans.1, 615, (1995).
- A. Y. Il’chenko, V. I. Krokhtyak, L. M. Yagupol’skii, Chem. Heterocycl. Compd. 17, 1236, (1981).
- K. Gobis, H. Foks, A. Kedzia, M. Wierzchowska, E. Kwapisz, Z. Zwolska, E. Augustynowicz-Kopec, Acta Pol Pharm. 63,39, (2006).
- W. Szczepankiewicz, J. Suwi’nski, R. Bujok: Tetrahedron 56, 9343, (2000).
- E. M. Cross, K. M. White, R. S. Moshrefzadeh, C.V. Francis, Macromolecules 28, 2526, (1995).
- K. G. Desai, K. R. Desai, Bioorg. Med. Chem. 14, 8271–8279, (2006).
- A. T. Mavrova, K. K. Anichina, D. I. Vuchev, J. A. Tsenov, P. S. Denkova, M. S. Kondeva, M. K. Micheva, Eur. J. Med. Chem. 41, 1412–1420, (2006).
- A. K. Singh, J. W. Lown, Anticancer Drug Des. 15, 265–275, (2000).
- P. Chaudhuri, B. Ganguly, S. Bhattacharya, J. Org. Chem. 72, 1912–1923, (2007).
- P. Hao, S. Zhang, W. -H. Sun, Q. Shi, S. Adewuyi, X. Lu, P. Li, Organometallics 26, 2439–2446, (2007).
- D. D. Rajadhyaksha, D. W. Rangnekar, J. Chem. Technol. Biotechnol. 36, 300–304, (1986).
- (a) N. Mehio, M. A. Lashely, J. W. Nugent, L. Tucker, B. Correia, C. L. Do-Thanh, S. Dai, R. D. Hancock, and V. S. Bryantsev, J. Phys. Chem. B 119, 3567–3576, (2015), (b) N. Mehio, B. Williamson, Y. Oyola, R. T. Mayes, C. Janke, S. Brown, and S. Dai, Ind. Eng. Chem. Res. 55, 4217– 4223, (2016).
- (a) X. Guo, Y. Wang, C.Li, P. Huai & G. Wu, Mol. Phys. 113, 1327-1336, (2015), (b) J. Xiong, S. Hu, Y. Liu, J. Yu, H. Yu, L. Xie, J. Wen, and X. Wang, ACS Sustainable Chem. Eng. 5, 1924–1930, (2017).
- O. Nibel, M. Bon, M. L. Agiorgousis, T. Laino, L. Gubler, and T. J. Schmidt, J. Phys. Chem. C 121, 6436–6445, (2017).
- S. Lin, , L. Yang, Tetrahedron Lett. 46, 4315, (2005).
- B. G. Zaleta, M. T. R. Silva, A. Guti´errez, E. G. Vergara, M. G. Rodr´ıguez, A. R. Hern´andez, Spectrochimic. Act. Part A 64, 1002, (2006).
- F. Eloy F, R. Lenaers, Chem. Rev. 62, 155, (1962).
- A. H. Amrallah, N. A. Abdalla, E. Y. El-Haty, J Chin. Chem. Soc. 53, 697, (2006).


