TEMPERATURE DEPENDENCE OF BILAYER PROPERTIES IN LIPOSOMES AND THE USE OF FLUORESCENT PROBES AS A TOOL TO ELUCIDATE THE PERMEATION MECHANISM OF HYDROPHILIC SOLUTES
- Liposome,
- Permeation,
- Glucose,
- Glucose oxidase,
- Fluorescent probe
Copyright (c) 2017 M. Ahumada, C. Calderon, E. A. Lissi

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
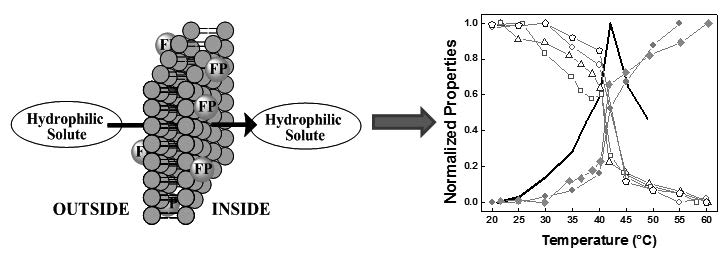
Solute transport across lipidic membranes is a fundamental process for both living organisms and drug delivery. In order to establish the mechanism of solute passive transport through lipidic membranes, we determined the effect of temperature upon the rate of glucose transport (as hydrophilic solute model) through dipalmitoylphosphatidylcholine large unilamellar liposomes and compare the results to those obtained for a variety of fluorescent probes (pyrene, PRODAN, diphenylhexatriene, diphenylhexatriene-TMA). All these probes, independent of their localization in the liposomes, report a monotonous change in the micro-properties sensed with temperature, with a maximum rate of change at the main transition temperature of the bilayer. These results contrast with those obtained for the rate of glucose influx, were it is observed a clear maximum rate of intake at the transition temperature. These contrasting results imply that the microviscosity (common factor for used probes) is not the property of the bilayer that controls the rate off solute transport which, at least in the vicinity of the main transition temperature, is regulated by formation of transient pores, particularly at the transition temperature (Tm). This would indicate that, at least near Tm, glucose and other hydrophilic solutes diffuses through transient pores formed in the bilayer. Interestingly, the presence of these pores does not affect the fluorescence characteristics of the probes.
Abbreviations
LUV Large unilamellar vesicles
GOx Glucose Oxidase
DPPC 1,2-Dipalmitoyl-sn-glycero-3-phosphatydylcholine
Tm Main phase transition temperatura
References
- K. Sugano, M. Kansy, P. Artursson, A. Avdeef, S. Bendels, L. Di, G.F. Ecker, B. Faller, H. Fischer, G. Gerebtzoff, H. Lennernaes, F. Senner. Coexistence of passive and carrier-mediated processes in drug transport. Nat Rev Drug Discov. 8, 597-614, 2010.
- M. Hediger, M. Romero, J-B. Peng, A. Rolfs, H. Takanaga, E. Bruford. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins. Plügers Arch.447, 465–468, 2004.
- A. Graff, M. Winterhalter, W. Meier. Nanoreactors from Polymer- Stabilized Liposomes. Langmuir 17, 919–923, 2001.
- S.Paula, A.G.Volkov, A.N.Van Hoek, T.H.Haines, D.W. Deamer. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys J. 70,339-348,1996.
- T.Shimanouchi, H. Ishii, N.Yoshimoto, H.Umakoshi, R.Kuboi.Calcein permeation across phosphatidylcholine bilayer membrane: Effects of membrane fluidity, liposome size, and immobilization. Colloids Surf B: Biointerfaces73, 156-160, 2009.
- M.Ahumada, C.Calderón, L.Leon, E.Lissi. Rate of solute incorporation to liposomes evaluated from encapsulated enzymes activities. Biophy. Rev.6, 161-167, 2014.
- S.Y.Hwang, H.K.Kima, J.Choo, G.H.Seong, T.B.DieuHien, E.K. Lee. Effects of operating parameters on the efficiency of liposomal encapsulation of enzymes. Colloids Surf B:Biointerfaces 94, 296–303, 2012.
- P.Walde, S. Ichikawa. Enzymes inside lipid vesicles: preparation, reactivity and applications. Biomol Eng.18, 143-77, 2001.
- V.Aswal, P.Goyal, S.Bhattacharya.Novel gemini micelles from dimeric surfactants with oxyethylene spacer chain. Small angle neutron scattering and fluorescence studies. J. Phys. Chem. B 102, 6152 – 6160, 1998.
- E.Abuin, E.Lissi, M. Ahumada. Diffusion of hydrogen peroxide across DPPC large unilamellar liposomes. ChemPhys Lipids 165, 656-661, 2012.
- W.V.Kraske, D.B. Mountcastle. Effects of cholesterol and temperature on the permeability of dimyristoylphosphatidylcholine bilayers near the chain melting phase transition. Biochim. Biophys. Acta 1514, 159 – 164, 2001.


