IMPROVEMENT OF THE ANTIFUNGAL ACTIVITY AGAINST BOTRYTIS CINEREA OF SYRINGIC ACID, A PHENOLIC ACID FROM GRAPE POMACE
- Botrytis cinerea,
- Antifungal activity,
- Laccase,
- 2,
- 6-dimethoxy-4-(phenylimino)benzenone
Copyright (c) 2017 Leonora Mendoza, Paulo Castro, Ricardo Melo, Ana María Campos, Gustavo Zúñiga, Juan Guerrero, Milena Cotoras

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
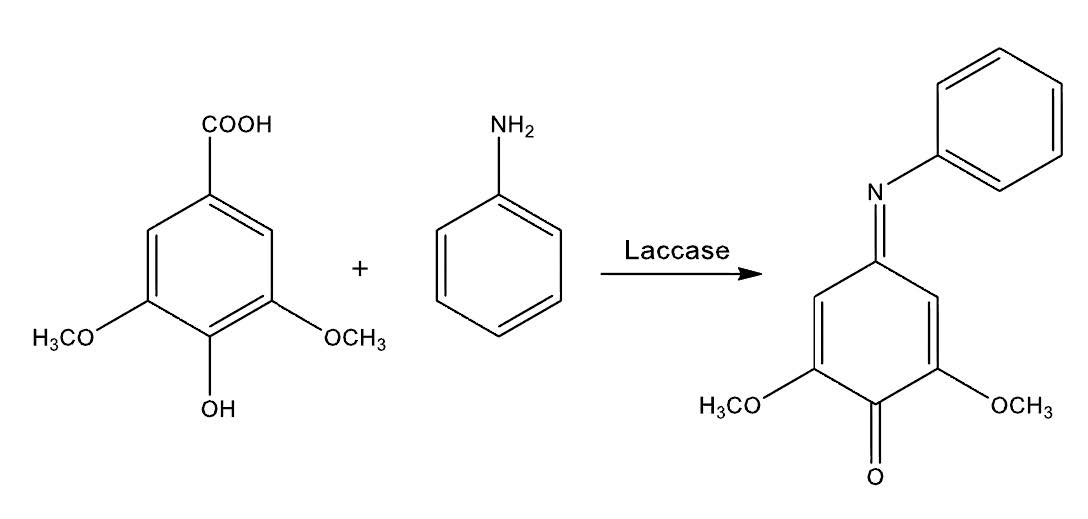
The aim of this study was to improve the antifungal activity against Botrytis cinerea of syringic acid by using the enzyme laccase to synthesize a heterodimeric compound by a coupling reaction with aniline. The synthesized heterodimer is a quinone-imine like compound (2,6-dimethoxy-4-(phenylimino)benzenone), which was characterized by using 1H and 13C NMR, IR, and mass spectrometry. The antifungal activity of the heterodimeric compound against B. cinerea was determined in vitro and showed a higher antifungal effect than the substrates (syringic acid and aniline), inhibiting the mycelial growth with an IC50 value of 0.14mM and delayed 2 hours the conidial germination. Also, using the fluorochrome calcofluor white, cell wall damage was observed when B. cinerea was incubated with the compound. This is the first report on antifungal activity of this type of compound against B. cinerea.
References
- Leroux, P. Chemical control of Botrytis and its resistance to chemical fungicides. In Botrytis: Biology, pathology and control; Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N., Eds.; Springer: Dordrecht, Netherlands, 2007; pp. 195–217.
- M. A. Jacometti, Wratten, S. D. Walter. Aust. J. Grape Wine Res. 2010, 16, 154–172.
- R. J. Grayer, J. B. Harborne, Phytochemistry 1994, 37, 19–42.
- E. Osbourn, Plant Cell 1996, 8, 1821–1831.
- J. B. Harborne, Biochem. Syst. Ecol. 1999, 27, 335–367.
- I. S. Arvanitoyannis, D. Ladas, A. Mavromatis, Int. J. Food Sci. Technol. 2006, 41, 475–487.
- L. Mendoza, K. Yañez, M. Vivanco, R. Melo, M. Cotoras, Ind. Crops Prod. 2013, 43, 360–364.
- A. Mikolasch, F. Schauer, Appl. Microbiol. Biotechnol. 2009, 82, 605–24.
- K. Manda, D. Gördes, A. Mikolasch, E. Hammer, E. Schmidt, K. Thurow, F. Schauer, Appl. Microbiol. Biotechnol. 2007, 76, 407–16.
- T. H. J. Niedermeyer, A. Mikolasch, M. Lalk, J. Org. Chem. 2005, 70, 2002–8.
- T. Kudanga, G. S. Nyanhongo, G. M. Guebitz, S. Burton, Enzyme Microb. Technol. 2011, 48, 195–208.
- J. Ihssen, M. Schubert, L. Thöny-Meyer, M. Richter, PLoS One 2014, 9, e89924.
- O. V. Morozova, G. P. Shumakovich, M. A. Gorbacheva, S. V. Shleev, A. I. Yaropolov, . Biochemistry 2007, 72, 1136–1150.
- U. Jonas, E. Hammer, E. T. K. Haupt, F. Schauer, Arch. Microbiol. 2000, 174, 393–398.
- T. Shiba, L. Xiao, T. Miyakoshi, C. Chen, J. Mol. Catal. B Enzym. 2000, 10, 605-615.
- O. E. Adelakun, T. Kudanga, A. Parker, I. R. Green, M. le Roes-Hill, S. G. Burton,. J. Mol. Catal. B Enzym. 2012, 74, 29–35.
- S. Witayakran, A. J. Ragauskas, Adv. Synth. Catal. 2009, 351, 1187–1209.
- V. Ibrahim, N. Volkova, S. H. Pyo, G. Mamo, R. Hatti-Kaul, J. Mol. Catal. B Enzym. 2013, 97, 45–53.
- Mikolasch, T. H. J. Niedermeyer, M. Lalk, S. Witt, S. Seefeldt, E. Hammer, F. Schauer, M. Gesell, S. Hessel, W. D. Jülich, U. Lindequist, Chem. Pharm. Bull. (Tokyo). 2006, 54, 632–638.
- Mikolasch, S. Hessel, M. G. Salazar, H. Neumann, K. Manda, D. Gordes, E. Schmidt, K. Thurow, E. Hammer, U. Lindequist, M. Beller, F. Schauer, D. Gōrdes, Chem. Pharm. Bull. 2008, 56, 781–786.
- Mikolasch, T. H. J. Niedermeyer, M. Lalk, S. Witt, S. Seefeldt, E. Hammer, F. Schauer, M. Gesell Salazar, S. Hessel, W.-D. Jülich, U. Lindequist, Chem. Pharm. Bull. (Tokyo). 2007, 55, 412–6.
- K. W. Wellington, N. I. Kolesnikova, Bioorg. Med. Chem. 2012, 20, 4472–81.
- G. Muñoz, P. Hinrichsen, Y. Brygoo, T. Giraud, Mycol. Res. 2002, 106, 594–601.
- L. Mendoza, P. Espinoza, A. Urzua, M. Vivanco, M. Cotoras, Molecules 2009, 14, 1966–1979.
- M. Cotoras, L. Mendoza, A. Muñoz, K. Yáñez, P. Castro, M. Aguirre, Molecules 2011, 16, 3885–3895.
- K. Thevissen, F. R. G. Terras, F. Willem, Appl. Environ. Microbiol. 1999, 65, 5451–5458.
- K. Tatsumi, A. Freyer, R. D. Minard, Soil Biol. Biochem. 1994, 26, 135– 142.
- J. M. Bollag, R. D. Minard, S. Y. Liu, Environ. Sci. Technol. 1983, 17, 72–80.
- Leonowicz, R. U. Edgehili, J. M. Bollag. Arch. Microbiol. 1984, 137, 89-96.
- H. Guenther, G. Jikeli, Chem. Rev. 1977, 77, 599–637.
- L. P. James, P. R. Mayeux, J. A. Hinson, Drug Metab. Dispos. 2003, 31, 1499–1506.
- Y. Jan, D. E. Heck, A. Dragomir, C. R. Gardner, D. L. Laskin, Chem. Res. Toxicol. 2014, 27, 882–894.
- L. Mendoza, R. Araya-Maturana, W. Cardona, T. Delgado-Castro, C. García, C. Lagos, M. Cotoras, J. Agric. Food Chem. 2005, 53, 10080–4.
- J. L. Bolton, M. Trush, T. M. Penning, G. Dryhurst, T. J. Monks, Chem. Res. Toxicol. 2000, 13, 135–60.
- W. Herth, E. Schnepf, Protoplasma 1980, 105, 129–133.


