LIGNANS IN OLIVE STONES DISCARDED FROM THE OIL INDUSTRY. COMPARISON OF THREE EXTRACTION METHODS FOLLOWED BY HPLC-DAD-MS/MS AND ANTIOXIDANT CAPACITY DETERMINATION
- lignans,
- pinoresinol,
- olive stones,
- Soxhlet,
- supercritical fluid extraction
- HPLC ...More
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Lignans are secondary metabolites with diverse chemical structure, and are frequent in food grains. A lignan rich diet have a positive health impact over chronic diseases and protection against certain types of cancer. To enhance the intake of lignans the addition of this compound in processed food could be a solution, so the best approach is to find a source of lignans, especially one that is discarded as waste. In the olive oil production there is a large volume of phytotoxic waste generated, among these is the olive stone (OS), that it is generally burned for fuel. It has been reported the presence of lignans in olive stones extracted with methanol reflux.
Three extraction methods for lignans and polyphenolic compounds were assessed: solid-liquid ultrasound assisted extraction with basic hydrolysis, methanolic extraction with Soxhlet, and CO2 supercritical fluid extraction with methanol as a modifier. The determination of lignan content in OS samples were evaluated by HPLC with a core shell C18 column and the detection was in tandem with DAD and MS/MS. The extracts were also evaluated in their antioxidant capacity with ABTS, CUPRAC and ORAC assays and total polyphenol concentration.
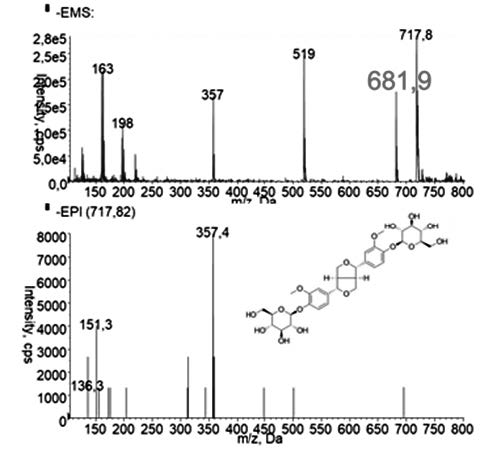
The aim of this work is to evaluate different methods to extract lignans from OS discarded from the olive oil industry. The main lignan identified in OS extract was pinoresinol. The SFE method to extract pinoresinol was more efficient than Soxhlet and ultrasound assisted solid liquid extractions, producing a higher amount of pinoresinol in the extract in less time and with less amount of co-extracted interferents. The extract produced with Soxhlet had a higher antioxidant capacity.
References
- N. Pellegrini, S. Valtuena, D. Ardigo, F. Brighenti, L. Franzini, D. Del Rio, F. Scazzina, P.M. Piatti, I. Zavaroni, Nutr. Metab. Cardiovasc. Dis. 20, 64, (2010).
- H. Adlercreutz, Crit. Rev. Clin. Lab. Sci. 44, 483, (2007).
- E. Roselló-Soto, M. Koubaa, A. Moubarik, R.P. Lopes, J.A Saraiva, N. Boussetta, N. Grimi, F.J. Barba, Trends Food Sci Technol. 45, 296, (2015).
- E. Antonini, A. Farina, E.S. Scarpa, A. Frati, P.Ninfali, Int J Food Sci Nutr, 67, 9, (2016).
- A. López-Biedma, C. Sánchez-Quesada, M. Delgado-Rodríguez, J.J. Gaforio, J Funct Foods. 26, 36, (2016).
- H.K. Obied, D.R. Bedgood, P.D. Prenzler, K. Robards, Anal. Chim, Acta, 603, 176, (2007).
- R.W. Owen, W. Mier, A. Giacosa, W.E.Hull, B. Spiegelhalder, H. Bartsch, Clin. Chem. 46(7), 976, (2000).
- J. Poerschmann, B. Weiner, I. Baskyr, Chemosphere, 92, 1472, (2013).
- A. Roig, M.L. Cayuela, M.A. Sanchez-Monedero, Waste Manag., 26, 960, (2006).
- R. Ghanbari, F. Anwar, K.M. Alkharfy, A.H. Gilani, N. Saari, Int. J. Mol. Sci. 13, 3291, (2012).
- M.J. Oliveras López, M. Innocenti, F. Ieri, C. Giaccherini, A. Romani, N. Mulinacci, J. Food Comp. Anal. 21, 62, (2008).
- A.B. Mansour, E.A. Porter, G.C. Kite, M.S.J. Simmonds, R. Abdelhedi, M. Bouaziz, J. Agric. Food Chem. 63, 1990, (2015).
- D. Boskou. Olive and Olive Oil Bioactive Constituents. Academic Press and AOCS Press, 2015.
- M.H. Alu’datt, I. Alli, K. Ereifej, M. Alhamad, A.R. Al-Tawaha, T. Rababah. Food Chem. 123, 117, (2010).
- I.E.J. Milder, I.C.W. Arts, D.P. Venema, J.J.P. Lasaroms, K.Wähälä, P.C.H. Hollman, J. Agric. Food Chem. 52, 4643, (2004).
- I.E.J. Milder, I.C.W. Arts, B. van de Putte, D.P. Venema, P.C.H. Hollman, Br. J. Nutr. 93, 393, (2005).
- S.M. Willfor, A.I. Smeds, B.R. Holmbom. J. Chromat. A, 1112, 64, (2006).
- M. Araújo, F.B. Pimentel, R.C. Alves, M.B.P.P. Oliveira, Trends Food Sci. Technol. 45, 200, (2015).
- C. Vergara, D. von Baer, C. Mardones, A. Wilkens, K. Wernekinck, A. Damm, S. Macke, T. Gorena, P. Winterhalter, J Agric Food Chem. 60, 929, (2012).
- C.N. Lin, A.M. Huang, K.W. Lin, T.C. Hour, H.H. Ko, S.C. Yang, Y.S. Pu, Phytochem. 71, 2140, (2010).
- J.P.N. Ribeiro, L.M. Magalhães, S. Reis, J.L.F.C. Lima, M. A. Segundo, Anal. Sci. 27, 483, (2011).
- B. Ou, H. Dejian, M. Hampsch-Woodill, J.A. Flanagan, E. K. Deemer, J. Agric. Food Chem. 50(11), 3122, (2002).
- M. Elbir, N.E. Es-Safi, A. Amhoud, M. Mbarki, Maderas Cienc Tecnol 17(3), 479, (2015).


