SYNTHESIS OF BENZOXAZEPINE DERIVATIVES FROM PYRAZOLE-CHALCONE VIA A SIMPLE AND CONVENIENT PROTOCOL USING BASIC ALUMINA AS SOLID SUPPORT
- Pyrazole,
- Chalcone,
- benzoxazepine,
- microwave irradiation,
- basic alumina
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
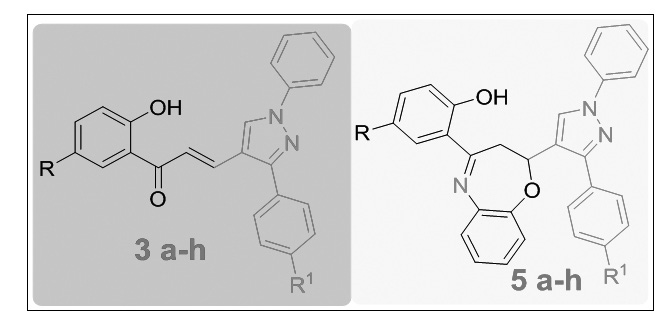
In the present study a series of novel benzoxazepine (5a-h) derivatives were synthesized by the thermal cyclization reaction of various pyrazole-chalcones (3a-h) with 2-aminophenol, by conventional heating and microwave irradiation (180 W) in solvent-free conditions in short reaction times (9–12 min), giving high yields of products (80–88%). The homogeneity of all the newly synthesized compounds has been checked by TLC. Their IR, NMR, ESI-mass spectral data and elemental analysis are in accord with the assigned structure. The title compounds were evaluated for their antibacterial activity against Gram-positive bacteria Staphylococcus aureus, Bacillus subtilis, and Gram-negative bacteria Pseudomonas aeruginosa, Escherichia coli. Compounds 3c, 3h, 5b, 5c, 5g and 5h were found to show good antibacterial activity when compared with that of standard drug Ampicillin. Furthermore, the same library of compounds were evaluated for antifungal activity against Aspergillus nigerzeae, Penicillium italicum and Fusarium oxysporum using Grieseofulvin as standard drug. The results of the above studies show that the compounds 3b, 3c, 3e, 3h, 5a, 5c, 5g and 5h showed good antifungal activity against all the tested organisms.
References
- E. Rohrmann, R.G. Jones, H.A. Shonle, J. Am. Chem. Soc., 66, 1856 (1944).
- G. Pasquale, G.P. Romanelli, J.C. Autino, J. García, E.V. Ortiz, P.R. Duchowicz, J. Agric. Food Chem., 60, 692, (2012).
- V. Monga, K. Goyal, M. Steindel, M. Malhotra, D.P. Rajani, S.D. Rajani, Med. Chem. Res., 23, 2019, (2014).
- F.E. Hawaiz, M.K. Samad, J. Chem., 3, 1613, (2012).
- K.A. Parmar, J.R. Vihol, Y.M. Dabhi, S.D. Sutariya, J. Chem. Pharm. Res., 4, 1584, (2012).
- O.A. Miqdad, N.M. Abunada, H.M. Hassaneen, A.S.M.A. Samaha, Int. J. Chem., 3, 20, (2011).
- V. Gressler, S. Moura, A.F.C. Flores, D.C. Flores, P. Colepicolo, E. Pinto, J. Braz. Chem. Soc., 21, 1477, (2010).
- M.A. Ali, M. Shaharyar, Bioorg. Med. Chem., 15, 1896, (2007).
- M. Shaharyar, A.A. Siddiqui, Bioorg. Med. Chem. Lett., 16, 3974, (2006).
- S. Bano, K. Javed, S. Ahmad, I.G. Rathish, S. Singh, M.S. Alam, Eur. J. Med. Chem., 46, 5763, (2001).
- K.M. Amin, S.M. Abou-Seri, F.M. Awadallah, A.A.M. Eissa, G.S. Hassan, M.M. Abdulla, Eur. J. Med. Chem., 90, 221, (2015).
- N.M.M. Moura, C. Nunez, S.M. Santos, M.A.F. Faustino, J.A.S. Cavaleiro, M.G.P.M.S. Neves, J.L. Capelo, C. Lodeiro, Inorg. Chem., 53, 6149, (2014).
- B.F. Abdel-Wahab, H. Abdel-Gawad, G.E.A. Awad, F.A. Badria, Med. Chem. Res., 1418, (2012).
- Y. Xia, Z.W. Dong, B.X. Zhao, X. Ge, N. Meng, D.S. Shin, J.Y. Miao, Bioorg. Med. Chem., 15, 6893, (2007).
- O.I. EI-Sabbagh, M.M. Baraka, S.M. Ibrahim, C. Pannecouque, G. Andrei, R. Snoeck, J. Balzarini, A.A. Rashad, Eur. J. Med. Chem., 44, 3746, (2009).
- C.N. Khobragade, R.G. bodade, B.S. Dawane, S.G. Konda, N.T. Khandare, J. Enzym. Inhib. Med. Chem., 25, 615, (2010).
- S. Sidique, R. Ardecky, Y. Su, S. Narisawa, B. Brown, J.L. Mill-an, E. Sergienko, N.D.P. Cosford, Bioorg. Med. Chem. Lett., 19, 222, (2009).
- J.F. Liegeois, M. Deville, S. Dilly, C. Lamy, F. Mangin, M. Resimont, F.I. Tarazi, J. Med. Chem., 5,1572, (2012).
- C.G. Neochoritis, C.A. Tsoleridis, J.S. Stephanatou, C.A. Kontogiorgis, D.J. Hadjipavlou-Litina, J. Med. Chem., 53, 8409, (2010).
- J.F. Liegeois, M. Deville, S. Dilly, C. Lamy, F. Mangin, M. Resimont, F.I. Tarazi, J. Med. Chem., 55, 1572, (2012).
- B. Capuano, I.T. Crosby, F.M. McRobb, D.A. Taylor, A. Vom, W.W. Blessing, Prog. Neuropsychopharmacol. Biol. Psychiatry, 34, 136, (2010).
- C.S. Takeuchi, B.G. Kim, C.M. Blazey, S. Ma, H.W.B. Johnson, N.K. Anand, A. Arcalas, T.G. Baik, C.A. Buhr, J. Cannoy, S. Epshteyn, A. Joshi, K. Lara, M.S. Lee, L. Wang, J.W. Leahy, J.M. Nuss, N. Aay, R. Aoyama, P. Foster, J. Lee, I. Lehoux, N. Munagala, A. Plonowski, S. Rajan, J. Woolfrey, K. Yamaguchi, P. Lamb, N. Miller, J. Med. Chem., 56, 2218, (2012).
- Y.L. Zhou, S.W. Shen, F.M. Liu, J. Heterocycl. Chem., 48, 105, (2011).
- N.D. Adhikary, P. Chattopadhyay, Eur. J. Org. Chem., 7346, (2011).
- P. Thansandote, E. Chong, K.O. Feldmann, M. Lautens, J. Org. Chem., 75, 3495, (2010).
- M. Larhed, C. Moberg, A. Hallberg, Acc. Chem. Res., 35, 717, (2002).
- S. Sarkar, S. Dutta, A.K. Sen, Synthesis 44, 1079, (2012).
- R. Pal, S. Sarkar, N. Chatterjee, A.K. Sen, Tetrahedron Lett., 54, 5642, (2013).
- P. Saha, S. Naskar, P. Paira, A. Hazra, K.B. Sahu, R. Paira, S. Banerjee, N.B. Mondal, Green Chem. 11, 931, (2009).
- H. J. Benson, Microbiological Applications, Boston, MA, U. S. A., W. C. Brown Publications, (1990).
- K. Saha, K. Mukherjee Pulok, S. C. Mandal, M. Pal, B. P. Saha, Indian Drugs, 32, 402, (1995).
- D. Ashok, G. Radhika, Indian J. Heterocycl. Chem., 20(3), 291, (2011).
- D. Ashok, A. Ganesh, V.B. Lakshmi, S. Ravi, Russ. J. Gen. Chem., 85(9), 2141, (2015).
- D. Ashok, D. Shravani, M. Sarasija, K. Sudershan, Russ. J. Gen. Chem., 85(5), 1152, (2015).
- D. Ashok, G. Sriniva, V.A. Kumar, D.M. Gandhi, M.S. Reddy, Russ. J. Gen. Chem., 85(3), 708, (2015).


