FACILE ECOFRIENDLY ONE POT SYNTHESIS OF HETEROCYCLIC PRIVILEDGED MEDICINAL SCAFFOLDS VIA BIGINELLI REACTION USING RETRIEVABLE NICKEL NANOPARTICLES AS CATALYST
- Nickel (II) exchanged zeolite [Ni(II)Y],
- Biginelli reaction,
- microwave irradiation,
- green condition,
- human pathogens
- DPPH radical scavenging activity ...More
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
An efficient and greener synthesis of a series of dihydropyrimidone (DHPMs)/ dihydropyrimithione (DHPMTs) derivatives were accomplished via three component one pot condensation between quinoline aldehyde (2-hydroxy-4-formyl quinoline/2-formyl-4-methoxy quinoline) , β – keto ester (ethyl acetoacetate / methyl acetoacetate) and urea/ thiourea using nickel(II) exchanged zeolite [Ni(II)Y] (NiNPs). The NiNPs were characterized by Infrared spectroscopy, Powder X ray diffraction patterns, Electronic microscopy studies- Scanning Electron Microscopy (FESEM) and Transmission Electron Microscopy (TEM). After the course of the reaction, the NiNPs were recovered and reused without any apparent loss of activity. The newly synthesized compounds were screened for antimicrobial activity against two human bacterial pathogens, the Gram-positive Methicillin resistant Staphylococcus aureus (MRSA) and the Gram-negative Pseudomonas aeruginosa (MTCC 201) and a human yeast pathogen, Fluconazole resistant Candida albicans (FRCA). The synthesised compounds were also evaluated for their antioxidant activity and the compounds show significant scavenging activity compared to aspartic acid.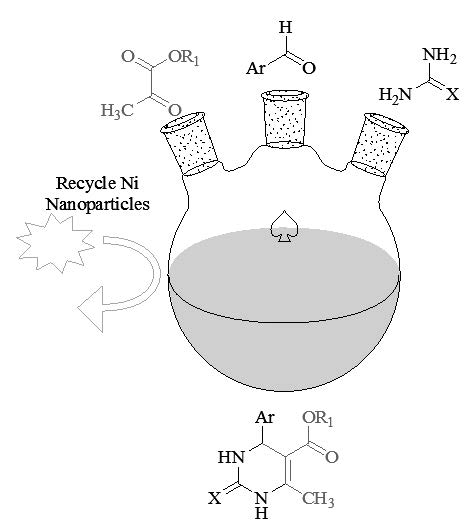
References
- A. Domling, Chem. Rev., 106(1),17 (2006).
- A. Domling and I. Ugi, Angew. Chem. Int. Ed, 39(18),3168 (2000).
- P. Biginelli, Gazz. Chim. Ital., 23,360 (1893).
- B.R.P. Kumar, G. Sankar, R.B.N. Baig and S. Chandrashekaran, E. J. Med. Chem., 44, 4192 (2009).
- M. Yarim, S. Sarac, M. Ertan, O. Batu and K. Erol, IL Farmaco, 54, 359 (1999).
- H. Nagarajaiah, A. Mukhopadhyay and J.N. Moorthy, Tetrahedron Lett. (2016), doi: http://dx.doi.org/10.1016/j.tetlet.2016.09.047
- a) S. Fatima, A. Sharma, R.Saxena, R. Tripathi, S.K. Shukla, S.K. Pandey, R. Tripathi and R.P. Tripathi, Eur. J. Med.Chem., 55, 195 (2012).
- b) H. Kaur, M. Machado, C. Kock, P. Smith, K. Chibale, M. Prudencio and K. Singh, Eur. J. Med.Chem., 101, 266 (2015).
- a) R.R. Joshi, A. Barchha, V.M Khedhar, R.R.S. Pissurlenkar, S. Sarkar, D. Sarkar, R.R. Joshi, R.A. Joshi, A.K. Shah and E.C. Coutinho, Chem. Biol. Drug. Des., 85, 201 (2015).
- b) N.C. Desai, A.R Trivedi and V.M. Khedkar, Bioorg. Med. Chem. Lett. (2016), doi: http://dx.doi.org/10.1016/j.bmcl.2016.06.082
- a) A. Crespo, A.E. Maatougi, P. Biagini, J. Azuaje, A. Coelho, J.M.I. Loza, M.I. Cadavid, X. Garcia- Mera, H. Gutierrez-de-Teran and E. Sotelo, ACS Med. Chem. Lett., 4, 1031 (2013).
- b) A.E. Maatougi, J. Azuaje, M. Gonzalez-Gomez, G. Miguez, A. Crespo, C. Carbajales, L.Escalante, X. Garcia-Mera, H.G. Teran and E. Sotelo, J. Med.Chem., 59, 1967 (2016).
- a) S. DeBonis, J.P. Simorre, I. Crevel, L. Lebeau, D.A. Skoufias, A. Blangy, C. Ebel, P. Gans, R. Cross, D.D. Hackney, R.H. Wade and F. Kozielsk, Biochemistry, 42, 338 (2003).
- b) H.Y.K. Kaan, V. Ulaganathan, O. Rath, H. Prokopcov, D. Dallinger, C.O. Kappe and F. Kozielski, J. Med. Chem., 53, 5676 (2010).
- a) K.S. Atwal, G.C. Rovnyak, S.D. Kimball, D.M. Floyd, S. Moreland, B.N. Swanson, J.Z. Gougoutas, J. Schwartz, K.M. Smillie and M.F. Malley, J. Med. Chem., 33, 2629 (1990).
- b) G.C. Rovnyak, K.S. Atwal, A. Hedberg, S.D. Kimball, S. Moreland, J.Z. Gougoutas, B.C. O’Reilly, J. Schwartz and M.F. Malley, J. Med. Chem., 35, 3254 (1992).
- D. Bozsing, P. Sohar, G. Gigler and G. Kovacs, Eur. J. Med. Chem., 31, 663 (1996).
- CO. Kappe, Eur. J. Med. Chem., 35, 1043 (2000).
- E.L.Khanina, G. Siliniece, J. Ozols, G. Duburs and A.Kimenis, Khim- Farm. Zh., 12, 72 (1978).
- a) T.N. Makarieva, K.M. Tabakmaher, A.G. Guzii, V.A. Denisenko, P.S. Dmitrenok, L.K. Shubina, A.S. Kuzmich H.S. Lee and V.A. Stonik, J. Nat. Prod., 74, 1952 (2011).
- b) Z.D. Aron and L.E. Overman, J. Am. Chem. Soc., 127, 3380 (2005).
- A.C. Boukis, A. Llevot and M.A.R. Meier, Macromol. Rapid Commum., 37, 643 (2016).
- Y. Zhao, Y. Yu, Y. Zhang, X. Wang, B. Yang, Q. Zhang, C. Fu, Y. Weia and L. Tao, Polym. Chem., 6, 4940 (2015).
- S.R. Patil, A.S. Choudhary, V.S. Patil and N. Sekar, Fibers Polym., 16, 2349 (2015).
- M. Puripat, R. Ramozzi, M. Hatanaka, W. Parasuk, V. Parasuk and K. Morokuma, J. Org. Chem., 80, 6959 (2015).
- H.G.O. Alvim, E.N. da Silva Junior and B.A.D. Neto, RSC Adv., 39(4), 54282 (2014).
- M.M. Heravi, S. Asadi and B.M. Lashkariani, Mol. Divers., 17, 389 (2013).
- M. Stucchi, G. Lesma, F. Meneghetti, G. Rainoldi, A. Sacchetti and A. Silvani, J. Org. Chem., 81, 1877 (2016).
- C.O.Kappe, Acc. Chem. Res., 33, 879 (2000).
- C. Zhu, B. Yang, Y. Zhao, C. Fu, L. Tao and Y. Wei, Polym. Chem., 4, 5395 (2013).
- Y. Ma, C. Qian, L.Wang and M.Yang, J. Org. Chem., 65, 3864 (2000).
- R. Ghosh, S. Maiti and A. Chakraborty, J. Mol. Catal. A, 217, 47 (2004).
- R.S. Bhosale, S.V. Bhosale, S.V. Bhosale, T. Wang and P.K. Zubaidha, Tetrahedron Lett., 45, 9111 (2004).
- W. Su, J. Li, Z. Zheng, Y. Ashen, Tetrahedron Lett., 46, 6037 (2005).
- V. Ch Reddy, M. Mahesh, R.V.K Raju, T. Ramesh Babu and V.V.N Reddy, Tetrahedron Lett., 43, 2657 (2002).
- S. Kumar, A. Saini and J.S. Sandhu, Indian J. Chem., 44B, 762 (2005).
- M.A. Pasha, N.R. Swamy, V.P. Jayashankara, Indian J Chem., 44B, 823 (2005).
- Y.V. Reddy, S. Kurva, S. Tammishetti, Catal. Commun., 5, 511 (2004).
- V.R. Choudhary, V.H. Tillu, V.S. Narkhede, H.B. Borate, R.D. Wakharkar, Catal. Commun., 4, 449 (2003).
- M. Tajbakhsh, B. Mohajerani, M.M. Heravi, A.N Ahmadi, J Mol. Catal. A, 236, 216 (2005).
- J. Lu,Y. Bai, Z. Wang, B. Yang, H. Ma, Tetrahedron Lett., 41, 9075 (2000).
- G. Maiti, P. Kundu, C. Guin Tetrahedron Lett., 44, 2757 (2003).
- M.M Heravi, F. Derikvand, F.F Bamoharram, J Mol Catal. A., 242, 173 (2005).
- F. Bigi, S. Carloni, B. Frullanti, R. Maggi, G. Sartori, Tetrahedron Lett., 40, 3465 (1999).
- P. Salehi, M. Dabiri, A.R. Khosropour, P. Roozbehniya, J Iran Chem Soc.,3, 98 (2006).
- S.L. Jain, V.V.D Prasad and B. Sain, Catal. Commun., 9, 499 (2008).
- G.K.Surya Prakash, H. Lau, C. Panja, I. Bychinskaya, S.K. Ganesh, B. Zaro, T. Mathew and G.A. Olah, Catal. Lett., 144, 2012 (2014).
- H. Kiyani and M. Ghiasi, Res Chem Intermed., (2014) http://doi: 10.1007/ s11164-014-1766-7.
- B. Vijayakumar, G.R. Rao, J Porous Mater., 19,491 (2012).
- H. El Badaoui , F. Bazi, S. Tamani ,S. Boulaajaj ,M. Zahouily ,H. B. Lazrek and S. Sebti, Synth Commun., 35, 2561 (2005).
- D.K Shete,S.S. Surve, S.B. Patil, S.B. Narade, K.S. Patil and Y.V. Pore, Der Pharm Lett 2, 59 (2010).
- H. El Badaoui, F. Bazi, S. Sokori, S. Boulaajaj, H.B. Lazrek and S. Sebti, Lett. Org. Chem., 2, 561 (2005).
- H. El Badaoui, F. Bazi, R. Tahir,H.B. Lazrek and S. Sebti, Catal. Commun., 6, 455 (2005).
- S. Sumathi and B. Gopal, Reac. Kinet. Mech. Cat., 117, 77 (2016).
- R. Varala, M. Mujahid Alam and S.R. Adapa, Synlett., 1, 67(2003).
- K. Ramalinga, P. Vijayalakshmi and T.N.B. Kaimal, Synlett., 6, 863 (2001).
- Y. Thirupathi Reddy, B. Rajitha, P. Narsimha Reddy, B. Sunil Kumar and V.P. Rao, Synth. Commun., 34, 3821 (2004).
- B.K. Banik, A.T. Reddy, A. Datta and C. Mukhopadhyay, Tetrahedron Lett., 48, 7392 (2007).
- M. Dewan, A. Kumar, A. Saxena, Arnab De and Mozumdar, Plos one., 7(8), 1 (2012).
- S.B. Sapkal, K.F. Shelke, B.B. Shingate and M.S. Shingare, Bull. Korean Chem. Soc., 31(2), 351 (2010).
- H.R.P Naik ,H.S.B Naik and T. Aravinda AJPAC 3(9), 202 (2009).
- D. Rossowsky, F.A. Lopes, V.S.S Da Silva, K.F.S Canto, M.G. Montes D’Oca and M.N. Godoi, J. Braz. Chem. Soc., 15(2), 165 (2004).
- J.H. Wang, E. Zhang, G.M. Tang, Y.T. Wang, Y.Z. Cui and S.W. Ng, J. Solid State Chem., 241, 86 (2016).
- H.M. Savanur, R.G. Kalkhambkar, G.Aridoss and K.K.Lalli, Tetrahedron Lett., 57, 3029 (2016).
- V. Poleshettiwar, B. Baruwati and R.S. Varma, Green Chem., 11 (1), 127 (2009).
- S.A. Majetich and Y. Jin, Science, 284, 470 (1999).
- L.K. Yeung and R.M. Crooks, Nano Lett., 1, 14 (2001).
- F. Alonso, P. Riente and M. Yus, Tetrahedron, 64(8), 1847 (2008).
- F. Alonso, P. Riente and M. Yus, Tetrahedron, 49, 1939 (2008).
- M. Kidwai, N.K. Mishra, V. Bansal, A. Kumar and S. Mozumdar, Catal. Commun., 9, 612 (2008).
- F. Alonso, P. Riente and M. Yus, Synlett., 9, 1289 (2008).
- F. Alonso, P. Riente and M. Yus, Eur. J. Org. Chem., 29, 4908 (2008).
- A. Saxena, A. Kumar and S. Mozumdar, J. Mol. Cat. A Chem., 269, 35 (2007).
- S.B. Sapkal, K.F. Shelke, B.B. Shingate and M.S. Shingare, Tetrahedron Lett., 50, 1754 (2009).
- G.G. Couto, J.J. Klein, W.H. Schreiner, D.H. Mosca , A.J.A. Oliveira and A.J.G Zarbin, J. Colloid. Interf. Sci., 311(2), 461 (2007).
- F.A Bassyouni, S.M. Abu Bakr and M.A. Rehin, Res. Chem. Intermed., 32(2), 283 (2012).
- R.S Varma (2002) In: Sumitra MK (ed) Astra Zeneca Research Foundation India, Bangalore, India. ISBN 81-901238-5-8.
- G.R. Reddy, K. Chennakesavulu and S. Balasubramanian, J.Mater. Chem. A.,2,15598 (2014).
- A. Murugesh, “Synthesis and biological evaluation of linear and angular fused napthyridines” Ph.D thesis, Bharathiar University, Coimbatore, Tamil Nadu, India, page 115 (2014).
- N.S. Kumar, S.P. Rajendran, As. J. Chem., 16(3-4), 1931(2004).
- M.R. Fazeli, G. Amin, M.M.A. Attar, H. Ashtiani, Jamalifar and H. Samadi, N. Food Control 18, 646(2007).
- Pa Wayne, National Committee for Clinical Laboratory Standards, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th Edition: Approved Standard M7-A5. National Committee for Clinical Laboratory Standards: Vol. 20, No. 2, 2000.
- H. Sini and K.S. Devi, Pharm Biol., 42, 462 (2004).
- C.O. Kappe, J.Org.Chem., 62, 7201 (1997).
- a) K. Folkers and T.B. Johnson, J. Am. Chem. Soc., 55, 3784 (1933).
- b) L.M. Ramos, A.Y.P.L. Tobio, M.R. Santos, H.C.B. Oliveria, A.F. Gomes, F.C. Gozzo, A.L. Oliveria and B.A.D. Neto, J.Org. Chem., 77, 10184 (2012).
- c) R. De Souza, E.T. Penha, H.M.S. Milagre, S.J. Garden, P.M. Esteves, M.N. Eberlin and O.A.C Anutunes, Chem. Eur. J., 15, 9799 (2009).
- S.S. Mansoor, S.S. Shafi and S.Z. Ahmed, Arabian J. Chem., (2016), doi: http://dx.doi.org/10.1016/j.arabjc.2011.09.0818
- A. Adhikari, B. Kalluyaya, K.V. Sujith, Gowthamchandra and K. Mahmood, Saudi Pharm. J., 20, 75 (2012).


