ONE-POT SYNTHESIS OF 2-ACYLAMINOBENZIMIDAZOLES FROM THE REACTION BETWEEN TRICHLOROACETYL ISOCYANATE AND 1,2-PHENYLENEDIAMINE DERIVATIVES AND THEORETICAL STUDY OF STRUCTURE AND PROPERTIES OF SYNTHESIZED 2-ACYLAMINOBENZIMIDAZOLES
- two-component reaction,
- 1,
- 2-phenylenediamine,
- trichloroacetyl isocyanate,
- heterocycle
- benzimidazol,
- GIAO method,
- NBO analysis ...More
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
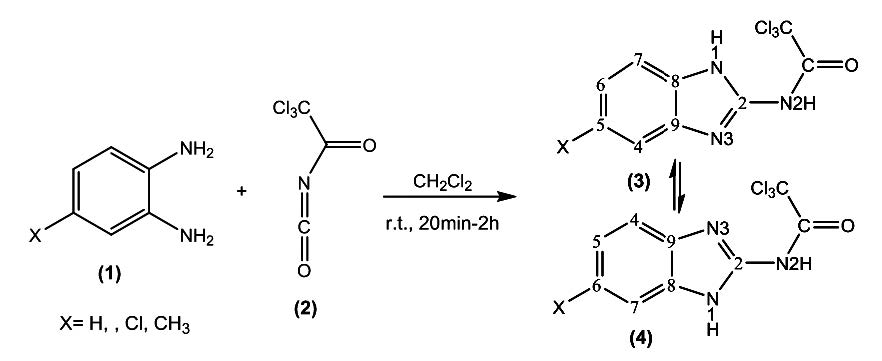
The two-component reaction of 1,2-phenylenediamine derivatives and trichloroacetyl isocyanate proceeded smoothly and cleanly at room temperature and N-(1H-benzimidazol-2-yl) acetamide derivatives afforded in excellent yields and no side reactions were observed. The structures of the products were confirmed by IR, 1H NMR, 13C NMR, and elemental analysis.
Also, in this investigation, the structural, electronic properties, IR, 13C and 1H NMR parameters of synthesized molecules were computed in gas in the M062X/6-311G(d,p) level of theory. 1H and 13C NMR chemical shifts were evaluated by employing of the gauge-invariant atomic orbital (GIAO) method. NBO analysis was exploited to examining of the atomic charges and their second order stabilization energy within these molecules.
References
- B.C. Bishop, E.T.J. Chelton, A.S. Jones, Biochem. Pharmacol, 13, 751 (1964).
- N. Boufatah, A. Gellis, J. Maldonado, P. Vanelle, Tetrahedron, 60, 9131 (2004).
- M.O. Chaney, P.V. Demarco, N.D. Jones, J.L. Occolowitz, J. Am. Chem. Soc., 96, 1932 (1974).
- M. Janssen, R.A. Stokbroekx, M. Luyckx, P.A. Janssen, J. Med. Chem. , 28, 1925 (1985).
- S. Sato, T. Kajiura, M. Noguchi, K. Takehana, T. Kobayasho, T. Tsuji, J. Antibiot., 54, 102 (2001).
- M. Ueki, K. Ueno, S. Miyadoh, K. Abe, K. Shibata, M.O.S. Taniguchi, J. Antibiot. , 46, 1089 (1993).
- I. Tamm, P.B. Sehgal, Adv. Virus. Res., 22, 186 (1978).
- I. Tamm, Science., 120, 847 (1954).
- M.M. Ramla, A.M. Omar, H. Tokudo, I.H. El-Diwoni, Bioorg. Med. Chem., 15, 6489 (2007).
- N.V. Gabrial, C. Roberto, H.C. Alicia, Y. Liian, H.L. Francisco, V. Juan, M. Rau, C. Rafael, H. Manuel, Bioorg. Med. Chem. Lett, 11, 187 (2001).
- P. Madsen, L.B. Knudsen, F.C. Wiberg, R.D. Carr, J. Med. Chem., 41, 5150 (1998).
- Z. Kazimierzcuk, D. Shugar, Nucleosides Nucleotides, 8, 1379 (1989).
- R. Ottana, S. Carotti, R. Maccari, I. Landini, G. Chiricosta, B. Cacigli, G.M. Vigorita, E. Mini, Bioorg. Med. Chem. Lett., 15, 3930.
- J.L. Perkins, A.E. Zartman, R.S. Meissner, Tetrahedron Lett. , 40, 1103 (1999).
- X. Wang, L. Zhang, Y. Zu, D. Krishnamurthy, C.H. Senanayake, Tetrahedron Lett, 45, 7167.
- A.-M.M.E. Omar, Synthesis, 41 (1974).
- U. Heinelt, D. Schultheis, S. Jager, M. .Lindermaier, A. Pollex, H.S.G. Beckmann, Tetrahedron, 60, 9883 (2004).
- A.-M.M.E. Omar, N.S. Habib, O.M. Aboulwafa, Synthesis, 864 (1977).
- V.J. Cee, N.S. Downing, Tetrahedron Lett, 47, 3747 (2006).
- R.D. Carpenter, P.B. DeBerdt, K.S. Lam, M.J. Kurth, J. Comb. Chem., 8, 907 (2006).
- N. Xie, F. Zhang, J. Li, X. Shi, Synlett, 6, 901 (2010).
- R.J. Snow, T. Butz, A. Hammach, S. Kapadia, T.M.Morwick, A.S. Prokopowicz, H. Takahashi, J.D. Tan, M.A. Tschantz, X.-J. Wang, Tetrahedron Lett., 43, 7553 (2002).
- K.S. Gudmundsson, J. Tidwell, N. Lippa, G.W. Koszalka, N.v. Draanen, R.G. Ptak, J.C. Drach, L.B. Townsend, J. Med. Chem., 43, 2464 (2000).
- J.C. Hazelton, B. Iddon, H. Suschitzky, L.H. Woolley, Tetrahedron, 51, 10771 (1995).
- B. Iddon, P. Kutschy, A.G. Robinson, H. Suschitzky, W. Kramer, F.A. Neugebauer, J. Chem. Soc., Perkin Trans., 1, 3129 (1992).
- N. Shajari, A.R. Kazemizadeh, A. Ramazani, J. Serb. Chem. Soc. , 77, 1175 (2012).
- A. Souldozi, A. Ramazani, Tetrahedron Lett., 48, 2617 (2007).
- A. Ramazani, N. Shajari, A.T. Mahyari, M. Khoobi, Y. Ahmadi, A. Souldozi, Phosphorus, Sulfur Silicon Relat. Elem., 185, 2496 (2010).
- A. Ramazani, N. Shajari, A. Mahyari, Y. Ahmadi, Mol. Divers., 15, 521 (2011).
- N. Shajari, A. Ramazani, Phosphorus, Sulfur Silicon Relat. Elem., 185, 1850 (2010).
- A. Ramazani, Y. Ahmadi, M. Rouhani, N. Shajari, A. Souldozi, Heteroatom Chemistry, 21, 368 (2010).
- A. Ramazani, B. Abdian, F.Z. Nasrabadi, N. Shajari, Z. Ranjdoost, Bull. Korean Chem. Soc, 33, 3701 (2012).
- A. Ramazani, M. Rouhani, A. Rezaei, N. Shajari, A. Souldozi, Helvetica Chimica Acta, 94, 282 (2011).
- A.R. Kazemizadeh, N. Hajaliakbari, R. Hajian, N. Shajari, A. Ramazani, Helvetica Chimica Acta, 95, 594 (2012).
- M. Rimaz, J. Khalafy, K. Tavana, K. Slepokura, T. Souldozi, A.T. Mahyari, N. Shajari, A. Ramazani, Z.Naturforsch, 64b, 1065 (2009).
- M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalman, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery, Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, O. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, in, Gaussian, Inc., Wallingford CT, 2009.
- R. Krishnan, J.S. Binkley, R. Seeger, A. Pople, J. Chem. Phys., 72, 650 (1980).
- Y. Zhao, D.G. Truhla, J. Phys. Chem, 110, 5121-5129 (2006).
- A.E. Reed, L.A. Curtiss, F. Weinhold, Chem. Rev. , 88, 899-926 (1988).
- E.D. Glendening, A.E. Reed, J.E. Carpenter, F. Weinhold, in.
- K. Wolinski, J.F. Hinton, P. Pulay, J. Am. Chem. Soc., 112, 8251–8260 (1990).
- V. Librando, A. Alparone, Z. Minniti, Journal of Molecular Structure: THEOCHEM, 856, 105 (2008).
- P.W. Ayers, R.G. Parr, J. Am. Chem. Soc., 422, 2010 (20000).
- R.G. Parr, P.K. Chattaraj, J. Am. Chem. Soc., 113, 1854 (1991).
- R.G. Pearson, J. Chem. Educ., 64, 561 (1987).
- R.G. Pearson, Acc. Chem. Res. , 26, 250 (1993).
- N.P.G. Roeges, A Guide to Complete Interpretation of IR Spectra of Organic Structures, Wiles, New York, 1994.
- N.B. Colthup, L.H. Daly, S.E. Wiberly, Introduction to Infrared and Raman Spectroscopy, Academic Press, New York, 1975.


