ONE POT PREPARATIONS N,N΄-ALKYLIDENE BISAMIDE DERIVATIVES CATALYZED BY NANO-TiCL4.SiO2 WITH ANTIMICROBIAL STUDIES OF SOME PRODUCTS
- Nano-TiCl4.SiO2,
- Heterogeneous catalyst,
- N,
- N’-Alkylidene bisamides,
- Antifungal
- Antibacterial ...More
Copyright (c) 2017 Soghra Khabnadideh, Kkamiar Zomorodian, Bi Bi Fatemeh Mirjalili, Elham Izadi, Leila Zamani

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
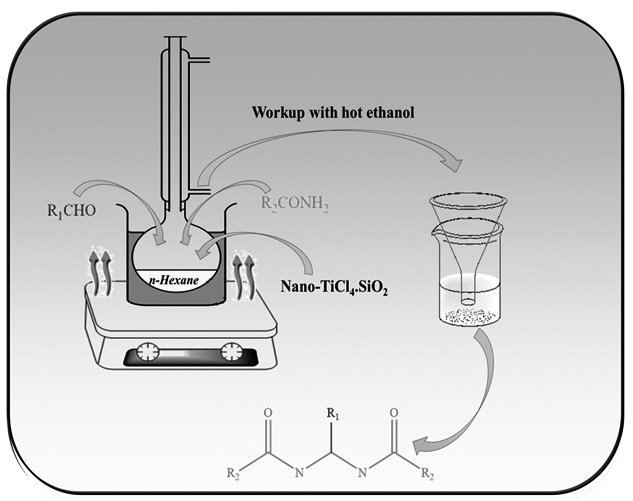
Nano-TiCl4.SiO2 has been introduced to be an extremely efficient catalyst for the preparation of N,N’-alkylidene bisamides from various aldehydes and amides under mild conditions. We synthesized this solid Lewis acid catalyst by the reaction of nano-SiO2 and TiCl4. The processor was simple and environmentally benign with high to excellent yields. Our method has the advantages of high yields, simple methodology, and easy work-up. The antimicrobial and antifungal activities of some of the synthetic compounds were determined by broth microdilution methods as recommended by Clinical Laboratory Standard Institute. Further studies still needed to investigate the other biological activities of the compounds.
References
- C. Aleman, J. Puiggali, J. Org. Chem., 60, 910, (1995).
- T. Yamazaki, K.I. Nunami, M. Goodman, Biopolymers, 31, 1513, (1991).
- M. Goodman, H. Shao, Pure & Appl. Chem., 68, 1303, (1996).
- P.V. Pallai, R.S. Struthers, M. Goodman, L. Moroder, E. Wunsch, W. Vale, Biochem., 24, 1933, (1985).
- M. Rodriguez, P. Dubreuil, J.-P. Bali, J. Martinez, J. Med. Chem., 30, 758, (1987).
- E.E. Magat, B.F. Faris, J.E. Reith, L.F. Salisbury, J. Am. Chem. Soc., 73, 1028, (1951).
- S. Zhu, G. Xu, Q. Chu, Y. Xu, C. Qui, J. Fluor. Chem., 93, 69, (1999).
- J. Pernak, B. Mrowczynski, J. Weglewski, Synthesis, 12, 1415, (1994).
- M.H. Mosslemin, M. Anary- Abbasinejad, A. Hassanabadi, S. Tajic, Synth. Commun., 40, 2209, (2010).
- R.M. Mohammad-Shafiee, Lett. Org. Chem., 8, 562, (2011).
- N.O. Brace, G.J. Mantell, J. Org. Chem., 26, 5170, (1961).
- Q. Wang, L. Sun, Y. Jiang, C. Li, L. Chunbao, Beilstein J. Org. Chem., 4, 51, (2008).
- G. Harichandran, S.D. Amalraja, P. Shanmugam, Indian J. Chem., 50 B (1), 77, (2011).
- G. Harichandran, S.D. Amalraja, P. Shanmugam, J. Iran. Chem. Soc., 8, 298, (2011).
- B. Maleki, M. Baghayeri, RSC Adv., 5, 79746, (2015).
- R. Pyrzehi-Bakhshani, A. Hassanabadi, J. Chem. Res., 3, 35, (2016).
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; approved stan¬dard. (2006), 2th edition. Wayne, PA: Clinical and Laboratory Standards Institute; CLSI M27-A7.
- Clinical and Laboratory Standards Institute (CLSI), “Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fun¬gi; Approved Standard,” Wayne, PA: Clinical and Laboratory Standards Institute, CLSI M38-A, (2006).
- B.F. Mirjalili, A. Bamoniri, L. Zamani, Scientia Iranica, C 19, 565, (2012).
- B.F. Mirjalili, A. Bamoniri, L. Zamani, Lett. Org. Chem., 9, 338, (2012).
- B.F. Mirjalili, L. Zamani, S. Afr. J. Chem., 67, 21, (2014).
- L. Zamani, B.F. Mirjalili, M. Namazian, Chemija 24, 312, (2013).
- L. Zamani, B.F. Mirjalili, K. Zomorodian, S. Zomorodian, S. Afr. J. Chem., 68, 133, (2015).
- L. Zamani, B.F. Mirjalili, Chem. Heterocycl. Compd., 51, 578, (2015).
- L. Zamani, B. F.Mirjalili, K. Zomorodian, M. Namazian, S. Khabnadideh, E. FaghihMirzaei, Farmacia, 62, 467, (2014).
- B.F. Mirjalili, L. Zamani, K. Zomorodian, S. Khabnadideh, Z. Haghighijoo, Z. Malakotikhah, S.A. Ayatollahi Mousavi, Sh. Khojasteh, J. Mol. Struct., 1116, 102 (2016).


