STABILITY STUDY AND VALIDATED REVERSED PHASE LIQUID CHROMATOGRAPHIC METHOD FOR THE DETERMINATION OF TIROFIBAN HYDROCHLORIDE IN PRESENCE OF TYROSINE AS A PROCESS IMPURITY
- Stability study,
- Tirofiban hydrochloride monohydrate,
- Tyrosine,
- Reversed-phase liquid chromatography,
- Method validation
- Intravenous infusion ...More
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
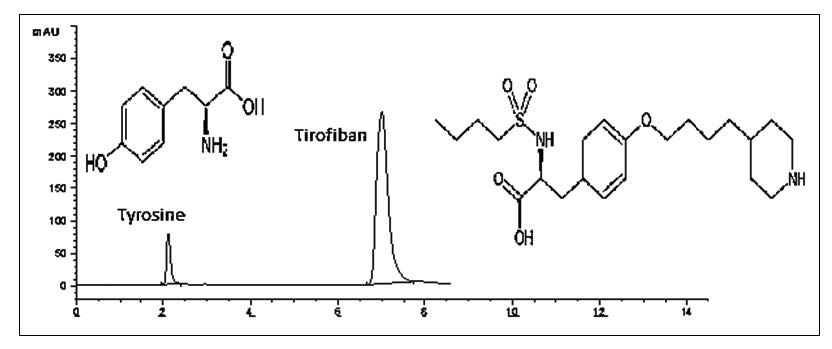
Tirofiban hydrochloride was subjected to the degradation under conditions of hydrolysis (acidic and alkaline degradation), oxidative, thermal and photolytic degradation as prescribed by ICH. A simple and precise liquid chromatographic method has been developed and validated for the simultaneous determination of tirofiban hydrochloride monohydrate (TIR) and its synthetic starting material; tyrosine (TRS). All the chromatographic separations were achieved on Zorbax SB C18, 250 mm×4.6 mm i.d., 5μm column at a flow rate of 1 mL min−1. Isocratic elution based on 0.1 M phosphate buffer (pH 3) - acetonitrile (70:30, v/v) with UV detection at 227 nm was applied. For the stability study separation of TIR from its degradation products was achieved using 0.1 M phosphate buffer (pH 3) - acetonitrile (72:28, v/v) with UV detection at 210 nm. Method validation parameters namely, linearity, accuracy and precision were found to be acceptable over the concentration ranges of 10-250 μg mL-1 for TIR and 1-70 μg mL-1 for TRS. The minimum detection limits were 1.76 μg mL-1 for TIR and 0.13 μg mL-1 for TRS. The optimized method was validated and proved to be specific, robust and accurate for the quality control of the cited drug in drug substance and drug product.
References
- Akl et al., J Chromat Separation Techniq, 4:4, (2013).
- Alison Brayfield, Martindale, The complete Drug Reference, 38th edn, P 2098, volume A, (2014).
- ZHENG Yong-Yong, ZHANG Zi-Xue, HUANG Lei et al, CJPH, 43(6): 408-410, (2012).
- HUANG Rong-fu, ZHAO Chi, SUN Jun-feng, WEI Qing, J. Cnki china pharmacy, (May 2010).
- M. Gandhimathi, Sai Theja Athoti and T.K. Ravi, IAJPR, 3(11): 9310- 9315, (2013).
- K. Sridevi Ranjitha and A.Lakshmana Rao, IJPCBS, 1(1), 43-47, (2011).
- Marija, et al. Int J Pharm, 4(4):115-120, (2014).
- YIN, Yang-jie; QIAO, Lei; ZHANG, Qi-ming , Chinese Journal of Pharmaceutical Analysis, Volume 33, pp. 1012-1015(4), Number 6, (2013).
- NATARAJ K.S, S.V. SAI KUMAR, N.V.V.S.KALYANI.P AND M. BADRU DUZA, ijpps, ISSN- 0975-1491 Vol 5, Suppl 1, (2013).
- Ellis JD, Hand EL and Gilbert JD, J Pharm Biomed Anal.,15(5):561-569, (1997).
- Oertel R, Kohler A, Koste A and Kirch W., J Chromatogr B., 805(1):181- 185, (2004).
- The European Agency for The Evaluation of Medical Products. ICH Topic Q2B Note for Guidance on Validation of Analytical Procedures: Methodology CPMP/ICH/281/95 (1996).
- In-house specification for the analysis of tirofiban, Gland chemical private LTD (Egypt).
- B.L. Agarwal, Basic statistics, 6 th edn, p. 189, 279, (2013).
- The United States Pharmacopoeia (USP 39), National Formulary (NF 34), Rockville, MD (2016).


