SYNTHESIS OF 5-HYDROXYDIHYDROPYRIMIDINE DERIVATIVES AND THE INFLUENCE OF INTRAMOLECULAR HYDROGEN BONDING ON THEIR NMR PROPERTIES AND CONFORMATIONAL PREFERENCES OF THE HYDROXYL GROUP
- hexahydropyrimidine,
- Mannich bases,
- hydrogen bonding,
- conformational preference
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
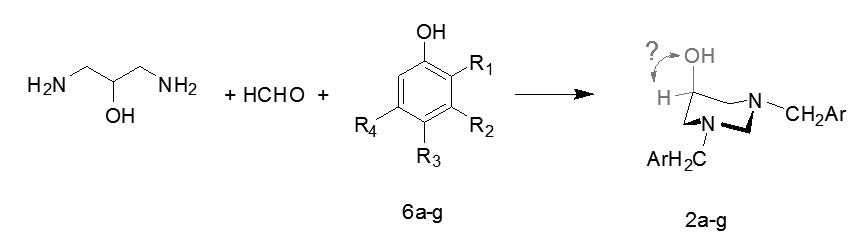
A series of 2,2’-(5-hydroxydihydropyrimidine-1,3(2H,4H)-diyl)bis-(methylene)-disubstitutedphenols was synthesized by using a Mannich-type reaction between 1,3-diaminopropan-2-ol, paraformaldehyde and substituted phenols. These previously unreported compounds were separated from the reaction mixture by column chromatography (CC) in highly pure form with a 29%-91% yield. The effect of intramolecular hydrogen bonding on the conformation of 5-hydroxyhexahydropyrimidines has been studied. Our results show that the presence of hydroxyl groups can strongly influence the stereoelectronic relationships in the six-membered heterocyclic ring.
References
- A. Rivera, J. Ríos-Motta, G.P. Trujillo, D.M. Gonzalez, D. Alcazar, Synthetic Commun. 43, 791, (2013).
- J.M. Locke, R.L. Crumbie, R. Griffith, T.D. Bailey, S. Boyd, J.D. Roberts, J. Org. Chem. 72, 4156, (2007).
- U. Salzner, J. Org. Chem., 60, 986, (1995).
- C. Garcías-Morales, S.H. Martínez-Salas, A. Ariza-Castolo, Tetrahedron Lett. 53, 3310, (2012).
- J. Dale, T. Sigvartsen, Acta Chem. Scand. 45, 1064, (1991).
- J.R. Farrell, J. Niconchuk, C.S. Higham, B.W. Bergeron, Tetrahedron Lett. 48, 8034, (2007).
- J.A. Bisceglia, J.E. Díaz, R.A. Torres, L.R. Orelli, Tetrahedron Lett. 52, 5238, (2011).
- T. Axenrod, J. Sun, K.K. Das, P.R. Dave, F. Forohar, M. Kaselj, N.J. Trivedi, R.D. Gilardi, J.L. Flippen-Anderson, J. Org. Chem. 65, 1200, (2000).
- D. Lin-Vien, N.B. Colthup, W.B. Fateley, J.G. Graselli, The handbook of Infrared and Raman characteristic frequencies of organic molecules. Academic Press: Boston, 1991.
- A. Rivera, I. Miranda-Carvajal, H.J. Osorio, J. Ríos-Motta, M. Bolte, Acta Cryst. E. 70, o687, (2014).
- A. Rivera, J. Ríos-Motta, R. Quevedo, P. Joseph-Nathan, Rev. Colomb. Quim. 34, 105, (2005).
- A. Rivera, G.I. Gallo, M.E. Gayón, P. Joseph-Nathan, Synth. Commun. 23, 2921, (1993).
- D. Danneels, M. Anteunis, Tetrahedron Lett. 16, 687, (1975).
- B. Brzezinski, G.S. Denisov, N.S. Golubev, S.N. Smirnov, J, Mol. Struct. 267, 383, (1992).


