- carbonated hydrocalumite,
- microwave radiation,
- antacid,
- carbonate calcium,
- aging times
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
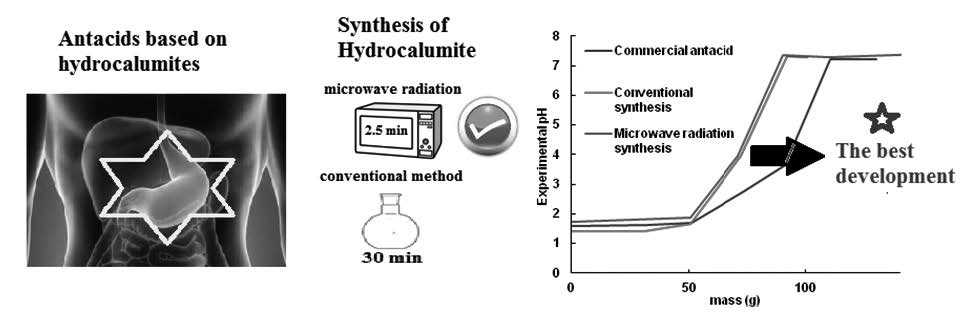
The synthesis of carbonated hydrocalumites could be troublesome because the carbonate anions can precipitate as calcium carbonate, therefore the aging times should be reduced. In this paper, carbonated hydrocalumites were synthesized using an unique gel divided in two portions: a part was aging to temperature room for 30 min, and the other one, by microwave radiations for 2 min. Samples were characterized by different techniques such as: Fourier-transformed infrared spectroscopy, X-ray diffraction, BET surface measurements, scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX). Then, they were tested as antacid using a synthetic gastric juice. Results showed that carbonated hydrocalumites aging by microwave radiations were more flawless than those obtained by the conventional method. Moreover, using microwave radiations, the aging times were notably reduced and the purity of carbonated hydrocalumites was improved. The antacid properties were similar for hydrocalumites synthesized by different ways. However, they showed they showed better antacid properties with higher antacid capacity than commercial antacid.
References
- M. Sánchez-Cantú, L.M. Pérez-Díaz, N. Tepale-Ochoa, V.J. González- Coronel, M.E. Ramos Cassellis, D. Machorro-Aguirre, J. Valente, Fuels 110, 23, (2013).
- M. Sánchez-Cantú, S. Camargo-Martínez, L.M. Pérez-Díaz, M. Hernández-Torres, E. Rubio-Rosas, J. Valente, Appl. Clay Sci. 114, 509, (2015).
- R. Segni, L. Vielle, F. Leroux, C. Taviot-Guèho, J. Phys. Chem. Solids, 67, 1037, (2006).
- E. López-Salinas, M.E. Llanos-Serrano, M.A. Cotés-Jácome, I. Schifter- Secora, J. Porous Mater. 2, 29, (1996).
- E. Pérez-Barrado, M. C. Pujol, M. Aguiló, J. Llorca, Y. Cesteros, F. Díaz, J. Pallarès, L.F. Marsal, P. Salagre, Chem. Eng. J. 264, 547, (2015).
- J. Cota, E. Ramírez, F. Medina, J.E. Sueiras, G. Layrac, D. Tichit, Appl. Clay Sci. 50, 498, (2010).
- M. Sipiczki, A.A. Ádám, T. Anitics, Catal. Today, 241, 231, (2015).
- M.A. Aramendía, B. Borau, C. Jiménez, J.M. Marinas, J. R. Ruiz, F.J Urbano, App. Catal., A: Gen. 206, 95, (2001).
- J. Kocík, M. Hájek, I. Troppová, Fuel Process. Technol. 134, 297, (2015).
- C.F. Linares, F. Ocanto, P. Bretto, M. Monsalve, Bull. Mater. Sci. 37, 341, (2014).
- M. Mora, M.I. López, C. Jiménez-Sanchidrián, J.R. Ruiz, J. R. Catal. Lett. 136, 192, (2010).
- E. Pérez-Barrado, M. C. Pujol, M. Aguiló, Y. Cesteros, F. Díaz, J. Pallarès, L.F. Marsal, P. Salagre, Appl. Clay Sci. 80-81, 313, (2013).
- J. Granados-Reyes, P. Salagre, P. Y. Cesteros, Y, Microporous Mesoporous Mater. 199, 117, (2014).
- M.H. Beers, R. Berkow, The Merk Manual of Diagnosis and Therapy, 10th ed., Merk: Madrid, 1999.
- A. Rivera, G. Rodríguez-Fuentes, E. Altshuler, Microporous Mesoporous Mater. 24, 51, (1998).
- M. Mora, M. I. López, C. Jiménez-Sanchidrián, J. R. Ruiz, Solid State Sci. 13, 101, (2011).
- S. Palmer, R. Frost, T. Nguyen, Coord. Chem. Rev. 253, 250, (2009).
- M. Campos, J. Santamaría, J. Mérida, R. Moreno, M. Alburquerque, S. Bruque-Gámez, E. Rodríguez-Castellón, A. Jiménez-López, P. Maireles- Torres, Energy Fuels 24, 979, (2009).
- K-H. Kok, T-T Lim, Z. Dong, Water Res. 42, 1343, (2008).
- M. Domínguez, M. Pérez-Bernal, R. Ruano-Casero, C. Barriga, V. Rives, R. Ferreira, L.D. Carlos; J. Rocha, Chem Mater. 23, 1993, (2011).
- R. Frost, S. Palmer, F. Theiss, J. Raman Spectrosc. 42, 1163, (2011).
- J. S.Fordtran, S. Morawski, C.T. Richardson, N. Engl. J. Med. 288, 923, (1973).


