DISPERSIVE LIQUID-LIQUID MICROEXTRACTION COUPLED WITH MICRO-VOLUME SPECTROPHOTOMETRY FOR DETERMINATION OF TOTAL IODINE IN URINE SAMPLES
- Urinary iodine,
- Methods comparison,
- Dispersive liquid-liquid microextraction
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
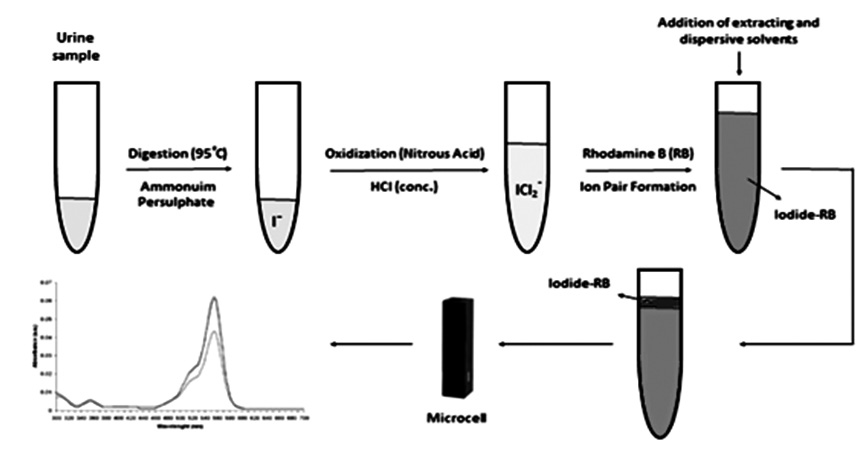
This work describes a simple photometric determination of iodine concentrations in urine using a dispersive liquid-liquid microextraction method. After digestion of the urine samples with ammonium persulfate, iodide was oxidized with nitrous acid to ICl2− anion at concentrated hydrochloric acid, then Rhodamine B is added and the ion-pair formed was extracted into a mixture of toluene (extracting phase) and ethanol (dispersive solvent) and measured spectrophotometrically. By means of this sensitive method, iodine concentrations can be determined in very small amount of urine specimens (250 μL). The calibration curve showed good linearity within the range of 100-900 μg.L-1 (R2 = 0.985), and the limit of detection for urine was 33.3 μg.L-1. The mean recovery of urine were 95.9-101.2 % with RSD of 2.6-6.6 %. The accuracy and precision of the method was evaluated by comparing to the standard Kolthoff-Sandell method. This suggested method affords a number of advantages, such as being simple, rapid, and inexpensive, with low organic solvent consumption, and is remarkably free from interference effects, rendering it an efficient method for the determination of iodine in urine samples in any routine clinical laboratory.
References
- T. T. Zava, S. Kapur, D. T. Zava, Anal. Chim. Acta. 764, 64-69, (2013).
- V. A. Arlington, Official methods of analysis of the association of official analytical chemists. 13th ed, AOAC. 1980; pp. 115.
- G. A. Kandhro, T. G. Kazi, N. Kazi, Sirajuddin, H. I. Afridi, M. B. Arain, G.A. Baig, A.Q. Shah, S. Khan, N.F. Kolachi, S.K. Wadhwa, F. Shah, Russ. J. Electrochem. 47, 1355-1362, (2011).
- C. Han, J. Sun, H. Cheng, Z. Xu, Anal. Methods. 6, 5369-5375, (2014).
- M. Dermeli, Z. Slejkovec, A. R. Byrne, P. Stegnar, S. Hojker, M. Porenta, G. Sestakov, Analyst. 117, 443-446, (1992).
- M. M. Mason, V. L. Spate, J. S. Morris, S. Chikos, C. K. Baskett, T. P. Cheng, C. L. Reams, L. O. Lemarchand, B. E. Henderson, L. N. Kolonel, Radioanal. Nucl. Chem. 195, 57-65, (1995).
- P. Allain, Y. Mauras, C. Douge, L. Jaunault, T. Delaporte, C. Beaugrand, Analyst. 115, 813-815, (1990).
- K. Mwaura, D. G. S. Narayana, A. M. Kinyua, J. Trace Elem. Electrolytes Health Dis. 8, 115-117, (1994).
- U. Divrikli, M. Soylak, M. Dogan, Chem. Anal-Warsaw. 45, 257-264, (2000).
- M. Kaykhaii, M. Sargazi, Spectrochim. Acta A. 121, 173-179, (2014).
- M. A. AL-Hajjaji, Anal. Chim. Acta. 197, 281-284, (1987).
- H. Yahyavi, M. Kaykhaii, M. Hashemi, RSC. Adv. 6, 2361-2367, (2016).
- M. R. Rezaei Kahkha, M. Kaykhaii, J. Chil. Chem. Soc. 60, 2986-2988, (2015).


