SYNTHESIS, CHARACTERIZATION AND UREASE INHIBITION STUDIES OF TRANSITION METAL COMPLEXES OF THIOUREAS BEARING IBUPROFEN MOIETY
- Amides,
- Thioureas,
- Metal complexes,
- Ibuprofen,
- Urease
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
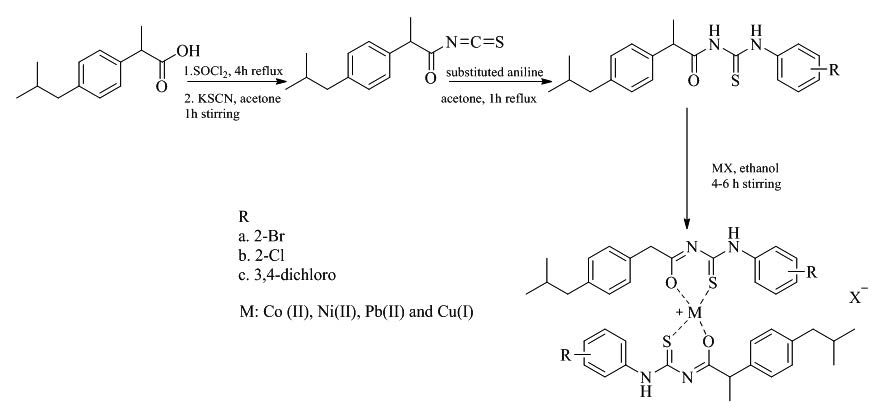
Starting from ibuprofen, a non-steroidal anti-inflammatory drug, N,N’-disubstituted thiourea derivatives were synthesized by refluxing the acid chloride of ibuprofen with potassium thiocyanate followed by substituted anilines to get N-2-(4-(2-methylpropyl)phenyl)propionoyl-N´-(2´-bromophenyl) thiourea (1), N-2- (4-(2-methylpropyl)phenyl)propionoyl-N´-(2´-chlorophenyl) thiourea (2) and N-2-(4-(2-methylpropyl)phenyl)propionoyl-N´-(3´,4´-dichlorophenyl) thiourea (3). Metal complexes (4-15) of (1-3) were synthesized by refluxing it with one equivalence salts of Co (ΙΙ), Ni(ΙΙ), Pb(ΙΙ) and Cu(Ι). Structures of all the synthesized thiourea ligands and their metal complexes were determined by FTIR and 1HNMR spectroscopy. Shift in stretching frequency in FTIR and resonance frequency in 1HNMR spectroscopic data suggested that the ligands and metal are coordinated to afford respective metal complexes M[L]n. The synthesized compounds were tested against urease enzyme and the results were compared with standard thiourea, as positive control. Most of the investigated compounds showed potential inhibitory activity against Jack bean urease. Compound 5 was found to be the most potent urease inhibitor with IC50 of 14.6 μM, whereas compounds 8, 11 and 12 possessed potent urease inhibition. The tested compounds can be taken as lead molecules for gastrointestinal ulcer therapy.
References
- Amtul Z RA, Siddiqui RA, Choudhary MI (2002) Chemistry and mechanism of urease inhibition. Curr Med Chem 9:1323-1348.
- Arslan H, Flörke U, Külcü N, Emen M (2006) Crystal structure and thermal behaviour of copper (II) and zinc (II) complexes with N-pyrrolidine-N′-(2- chloro-benzoyl) thiourea Journal of Coordination Chemistry 59:223-228.
- Aslam MA MS, Shahid M, Saeed A, Iqbal J (2011) Synthesis, biological assay in vitro and molecular docking studies of new Schiff base derivatives as potential urease inhibitors. Eur J Med Chem 46:5473-5479 doi:doi: 10.1016/j.ejmech.2011.09.009.
- Bacanamwo M WC-P, Lubbers M, Polacco J, (2002) Activation of the urease of Schizosaccharomyces pombe by the UreF accessory protein from soybean. Mol Genet Genomics 268:525-534.
- Benini S (1999) A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Structure 7:205.
- Beyer L HE, Hennig H, Kirmse R, Hartmann H, Liebscher J (1975) Synthese and charakterisierung neuartiger übergangsmetallchelate von 1,1-dialkyl-3-benzoyl-thioharnstoffen J Prakt Chem 317:829 – 839.
- Bundy LG BJ (1973) Effects of substituted p-benzoquinones on urease activity in soils. Soil Biol Biochem 5:847-853.
- Carcu V NM, Rosu T, Serban S (2000) Synthesis, Characterization of Complexes of N-benzoyl-n’-2-nitro-4-methoxyphenyl-thiourea with Cu, Ni, Pt, Pd, Cd and Hg. J Therm Anal Calorim 61:935-945 doi:1023/A:1010163012703.
- Costa C, Gushikem Y (1984) Interaction of N-acylthiourea nickel (II) chelates with nitrogen bases in solution and in the solid state. Inorganica chimica acta 90:133-136.
- D’Cruz OJ DY, Uckun FM, (2003) Potent dual anti-HIV and spermicidal activities of novel oxovanadium(V) complexes with thiourea non-nucleoside inhibitors of HIV-1 reverse transcriptase Biochemical and biophysical research communications 302:253-264.
- Del CR CJ, Garcı́a E, Hermosa RR, Jiménez-Sánchez A, Manzano JL, Monte E, Rodrı́guez-Fernández E., Sanz F (2002) Thiourea derivatives and their nickel(II) and platinum(II) complexes: antifungal activity. J Inorg Biochem 89:74-82 doi:http://dx.doi.org/10.1016/S0162-0134(01)00408-1.
- Dhumane NR HS, Nawarkhele VV, Shirsat MD, (2006) Dielectric studies of metal complexes of thiourea crystals for electro‐optic modulation. Cryst Res Technol 41:897-901.
- Douglass IB, Dains FB (1934) Some derivatives of benzoyl and furoyl isothiocyanates and their use in synthesizing heterocyclic compounds. J Am Chem Soc 56:719-721.
- Gripenberg-Lerche C ZL, Ahtonen P, Toivanen P, Skurnik M (2000) Construction of urease-negative mutants of yersinia enterocolitica serotypes o: 3 and o: 8: role of urease in virulence and arthritogenicity. Infect Immun 68:942-947.
- Hakan A NK, Ulrich F (2003) Synthesis and characterization of copper(II), nickel(II) and cobalt(II) complexes with novel thiourea derivatives. Transition Met Chem 287:816-819.
- Hanif M SK, Saleem M, Rama NH, Zaib S, Iqbal J (2012) Synthesis, urease inhibition, antioxidant, antibacterial, and molecular docking studies of 1, 3, 4-oxadiazole derivatives. ISRN pharmacology 2012 doi:doi. org/10.5402/2012/928901.
- Holm LS, Chris, (1997) Proteins Struct Funct Genet 28:72-82.
- KD T (2003) Drugs for peptic ulcer. In: In Essentials of Medical Pharmacology. 5th edn. Jaypee Brothers Medical Publishers, New Delhi, pp 587-598.
- Kobashi K HJ, Uehara K (1962,) Specific inhibition of urease by hydroxamic acids. Biochim Biophys Acta 65:380.
- Kot M ZW, Orlinska K (2001) Inhibition of jack bean urease by N-(n-butyl) thiophosphorictriamide and N-(n-butyl) phosphorictriamide: determination of the inhibition mechanism J Enzyme Inhib 16:507-516.
- Krajewska B (2009) Ureases I. Functional, catalytic and kinetic properties: A review. J Mol Catal B Enzym 59:9-21.
- Kuehler TC FJ, Bergman NA, Weilitz J, Lee A, Larsson H (1995) Structure-activity relationship of omeprazole and analogs as Helicobacter pylori urease inhibitors J Med Chem and Res 38:4906-4916.
- Kumavat P, Jangale AD, Patil DR, Dalal KS, Meshram JS, Dalal DS (2013) Green synthesis of symmetrical N, N′-disubstituted thiourea derivatives in water using solar energy. Environ Chem Lett 11:177-182.
- Kumavat PP JA, Patil DR, Dalal KS, Meshram JS, Dalal DS (2013) Green synthesis of symmetrical N, N′-disubstituted thiourea derivatives in water using solar energy. Environ Chem Lett 11:177-182.
- Losada J PI, Beyer L (2000) Synthesis, electrochemical properties and electro-oxidative polymerizationof copper(II) and nickel(II) complexes with N’-benzoylthiourea ligands containing pyrrole. Transition Met Chem 25:112-117.
- McMillan DC LE, Smith J, Sturgeon C, Preston T, Cooke TG, McArdle CS, (1995) Effect of extended ibuprofen administration on the acute phase protein response in colorectal cancer patients. Eur J Surg Oncol 21:531- 534.
- Mobley H, Island MD, Hausinger RP (1995) Molecular biology of microbial ureases Microbiological reviews 59:451-480.
- Mobley HL HR (1989) Microbial ureases: significance, regulation, and molecular characterization Microbiol Rev 53:85-108.
- Moro AC MA, Netto AV, Ananias SR, Quilles MB, Carlos IZ, Pavan FR, Leite CQ, Hoer M (2009) Antitumor and antimycobacterial activities of cyclopalladated complexes: X-ray structure of [Pd(C2,N-dmba)(Br) (tu)] (dmba=N,N-dimethylbenzylamine, tu=thiourea). Eur J Med Chem 44:4611-4615.
- Muri EMF MH, Avery MA Williamson JS (2003) Design and Synthesis of Heterocyclic Hydroxamic Acid Derivatives as Inhibitors of Helicobacter pylori Urease. Synthetic Commun 33:1977.
- Nadeem S RM, Ahmad S, Ebihara M, Tirmizi SA, Bashir SA, Badshah A (2009) Synthesis and characterization of palladium (II) complexes of thioureas. X-ray structures of [Pd (N, N′-dimethylthiourea) 4] Cl2· 2H2O and [Pd (tetramethylthiourea) 4] Cl2 Transition Met Chem 34:197-202.
- Nagata K SH, Iwahi T, Shimoyama T, Tamura T (1993) Potent inhibitory action of the gastric proton pump inhibitor lansoprazole against urease activity of helicobacter pylori: unique action selective for H. pylori cells. Antimicrob Agents Chemother 37:769-774.
- Rao P Kea (2008) Evolution of nonsteroidal anti-inflammatory drugs (nsaids): Cyclooxygenase (cox) inhibition and beyond. J Pharm Pharm 11:81-110.
- Rasmussen CR VFJ, Weaner, Reynolds BE, Hood AR, Hecker LR, Nortey SO, Hanslin A, Costanzo MJ, Powell ET, Molinari AJ (1988) Improved procedures for the preparation of cycloalkyl, arylalkyl and arylthioureas. Synthesis 6:456–459 doi:10.1055/s-1988-27605.
- Rauf MK BA, Bolte M, Imtiaz-ud-Din (2006) 1-(2,6-Dichlorobenzoyl)- 3-(2-chlorophenyl) thiourea toluene hemi solvate. Acta Crystallogr E62:o3553.
- Rauf MK BA, Bolte M, Imtiaz-ud-Din (2010) Solution-phase synthesis of a combinatorial n, n’-disubstituted thioyrea library: Complexation, characterization and biological studiest. Quaid-i-Azam University, Islamabad.
- Rauf MK BA, Gielen M, Ebihara M, Vos D, Ahmed S (2009) Synthesis, structural characterization and in vitro cytotoxicity and anti-bacterial activity of some copper(I) complexes with N,N’-disubstituted thioureas. J Inorg Biochem 103:1135-1144 doi:10.1016/j.jinorgbio.2009.05.014.
- Rauf MK SA, Bolte M, Badshah A, Mirza B, (2008) Synthesis, characterization and biological activities of some new organotin(IV) derivatives: Crystal structure of [(Sn Ph3) (OOCC6H4OH)] and [(SnMe3)2 (OOC)2C6Cl4 (DMSO)2]. J Organomet Chem 693:3043-3048.
- Saeed A, Khan MS, Rafique H, Shahid M, Iqbal J (2014) Design, synthesis, molecular docking studies and in vitro screening of ethyl 4-(3-benzoylthioureido) benzoates as urease inhibitors. Bioorganic chemistry 52:1-7.
- Saeed A, Zaib S, Pervez A, Mumtaz A, Shahid M, Iqbal J (2013) Synthesis, molecular docking studies, and in vitro screening of sulfanilamide-thiourea hybrids as antimicrobial and urease inhibitors J Med Chem and Res 22:3653-3662.
- Sun MZ WF, Wu YM, Liu WM (2008) A ditopic colorimetric sensor for fluoride ion based on thiourea mercury complex. Spectrochim Acta 71:814-817.
- Todd MJ HR (1989) Competitive inhibitors of Klebsiella aerogenes urease. Mechanisms of interaction with the nickel active site J Biol Chem 264:15835-15842.
- Tremblay L DG, Ghali E, McMullen J, Lanouette M, (1996) Gold recovery from a sulfide bearing gold ore by percolation leaching with thiourea. Int J Miner Process 48:225-244.
- Weatherburn M (1967) Phenol-hypochlorite reaction for determination of ammonia Analytical chemistry 39:971-974.
- Yuan YF WJ, Gimeno MC, Laguna A., Jones PG, (2001) Synthesis and characterisation of copper complexes with N-ferrocenoyl-N ‘(alkyl) thioureas Inorg Chimica Acta 324:309-317.


