LACASSE CATALYZED-SYNTHESIS OF 4,4’-BIPHENYLDIAMINE FROM P-CHLOROANILINE. EVALUATION OF ANTIFUNGAL AND ANTIOXIDANT ACTIVITIES
- P-chloroaniline,
- 4,
- 4’-Biphenyldiamine,
- Laccase,
- Antioxidant activity
- Antifungal activity ...More
Copyright (c) 2017 Ana M. Campos, Leonora Mendoza, Juan Vásquez, Ricardo Melo, Jennifer Salas, Milena Cotoras

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
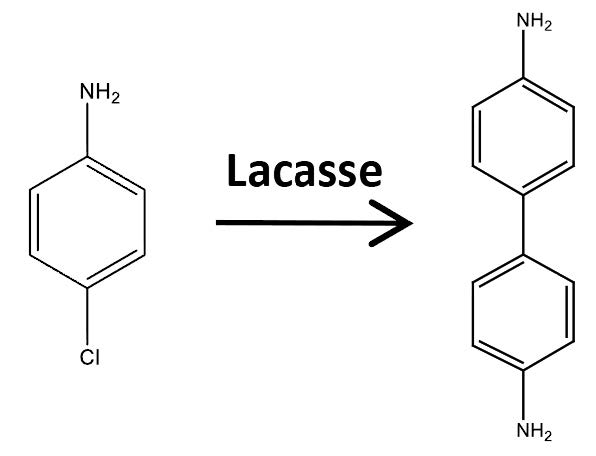
Laccase is a copper-containing oxidase that catalyzes reduction of molecular oxygen to water and the oxidation of a phenolic compound. In this paper, laccase was utilized to synthesize 4,4’-Biphenyldiamine using p-chloroaniline as substrate by means of a coupling reaction. The synthesized compound, 4,4’-Biphenyldiamine, presented low antifungal activity against the phytopathogenic fungus Botrytis cinerea, however the antioxidant ability, measurement by ORAC-PGR method, was higher than substrate.
This work corresponds to the first report of synthesis 4,4’-Biphenyldiamine, from p-chloroaniline in a lacasse-catalyzed reaction.
References
- O.V. Morozova, G.P. Shumakovich, M.A. Gorbacheva, S.V. Shleev, A. Yaropolov, Biochem.72, 1136, (2007).
- T. Kudanga, G.S. Nyanhongo, G.M. Guebitz, S. Burton, Enz. Microbial Tech. 48, 195, (2011).
- U. Jonas, E. Hammer, E.T.K. Haupt, F. Schauer, Arch. Microbiol.174,393 (2000).
- T. Shiba, L. Xiao, T. Miyakoshi, C. Chen, J. Mol. Catal. B. Enzym, 10(6), 7 (2000).
- O.E. Adelakun,T. Kudanga,A. Parker,I.R. Green, M. le Roes-Hill, S.G Burton,. J. Mol. Catal. B Enzym.74, 29, (2012).
- S. Witayakran,A.J. Ragauskas, Adv. Synth. Catal.351, 1187, (2009).
- T.H.J. Niedermeyer, A. Mikolasch, M. Lalk, J. Org. Chem.70, 2002 (2005).
- V. Ibrahim, N. Volkova,S.H. Pyo, G. Mamo, R. Hatti-Kaul, Acid. J. Mol. Catal. B Enzym.97, 45, (2013).
- Mikolasch, T.H.J. Niedermeyer, M. Lalk, S. Witt, S. Seefeldt, E. Hammer, F. Schauer, M. Gesell, S. Hessel, W.D. Jülich, et al. Chem. Pharm. Bull. (Tokyo).54, 632, (2006).
- Mikolasch, S. Hessel, M.G. Salazar, H. Neumann, K. Manda, D. Gordes, E. Schmidt, K. Thurow, E. Hammer, U. Lindequist, et al. Chem. Pharm. Bull.56, 781, (2008).
- Mikolasch, T.H.J. Niedermeyer, M. Lalk, S. Witt, S. Seefeldt, E. Hammer, F. Schauer, M. Gesell Salazar, S. Hessel, W.D. Jülich, et al. Chem. Pharm. Bull. (Tokyo).55, 412, (2007).
- K.W. Wellington, N.I. Kolesnikova, Bioorg. Med. Chem.20, 4472, (2012).
- J.R. Jeon, Y.S. Chang, Trends in Biotechnology, 31(6), 335, (2013).
- G. Shumakovich, V. Kurova, I. Vasil’eva, D. Pankratov, G. Otrokhov, O. Morozova, A. Yaropolov, J. Mol. Catal. B. Enzym. 77,105, (2012).
- M. Kurisawa, J. Chung, H. Uyama, S. Kobayashi, Biomacromolecules, 4, 1394 (2003).
- M.G. Hossain, M.D. González, G.R. Lozano, T. Tzanov, J. Biotech. 141, 58 (2009).
- A.A.Leontievsky, N.M. Myasoedova, B.P. Baskonov, L.A. Golovleva, C.S. Evans. Biodegradation 11(5), 331, (2000).
- G. Dawel, M. Kastner, J. Michels, W. Poppitz, W. Gunther, W. Fritsche, Applied Environ. Microbiol. 63(7), 2560, (1997).
- J. F. Osma, J. Toca-Herrera, S. Rodriguez-Couto, Dyes & Pigments, 75, 32, (2007).
- Cantarelli, Vinid’Italia, 3, 87, (1986).
- M. Božiča, J. Štrancarb, V. Kokola, Reac. Func. Polymers 73(10), 1377, (2013).
- Aljawish, I. Chevalot J. Jasniewski, A.M. Revol-Junelles, J. Scher, J. Muniglia, Food Chem. 161, 279, (2014).
- P. Leroux in the Chemical Control of Botrytis and Its Resistance to Chemical Fungicides. In Botrytis: Biology, pathology and control; Y. Elad, B. Williamson, P. Tudzynski, N. Delen, N., Eds.; Springer: Dordrecht, Netherlands, 2007; pp. 195–217.
- G. Muñoz, P. Hinrichsen,Y. Brygoo, T. Giraud, Mycol. Res.106, 594, (2002).
- A.C. Poblete, B. E. López-Alarcón, C. Lissi, A.M. Campos, J. Chil. Chem. Soc. 54(2), 14, (2009).
- R. Silverstein, F. Webster, D. Kiemle. Spectrometric identification of organic compounds, John Wiley & Sons, (2005).
- Hinks, H.S. Freemana, M. Nakpathoma, J. Sokolowskab, (2000). Dyes and Pigments, 44(3), 199, (2000).
- T. Hoff, S.Y. Liu, J.M. Bollag, App. Environ. Microbiol. 49 (5), 1044, (1985).
- W.G. Levine, W. G. in the Laccase, a review, In J.J. Peisach, D. Aisen, and W.E. Blumberg, ed. The biochemistry of copper. Academic Press, Inc., New York, (1966); p. 371-387.
- K.E. Simmonds, R.D. Minard, J.M. Bollag, Environ. Sci. Technol. 27, 999, (1987).
- Longoria, H. Hub, R. Tinoco-Valencia, R. Vásquez-Duhalt, TIP Revista Especializada en Ciencias Químico-Biológicas, 11(2), 73, (2008).
- L. Mendoza, M. Cotoras, M. Vivanco, B. Matsuhiro, S. Torres, M.J. Aguirre, J. Chil. Chem. Soc. 58(2), 1725, (2013).
- M. Adrian, P. Jeandet, J. Veneau, L. Weston, R. Besis, J. Chem. Ecol. 23, 1689, (1997).
- F. Caruso, L. Mendoza, P. Castro, M. Cotoras, M.J. Aguirre, B. Matsuhiro, M. Isaacs, M. Rossi, A. Viglianti, R. Antonieletti, PLOS-ONE 6(10), e25421, (2011).
- A.Schouten, L. Wagemakers, F.L. Stefanato, R.M. Van der Kaaij, J.A.L van Kan, Mol. Microbiol. 43, 883, (2002).


