- Carbon steel,
- Green corrosion inhibitors,
- Date extract,
- Electrochemical methods,
- Acid medium
Copyright (c) 2017 Naima Moussaoui, Djilali Zerouali, Noureddine Bettahar

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
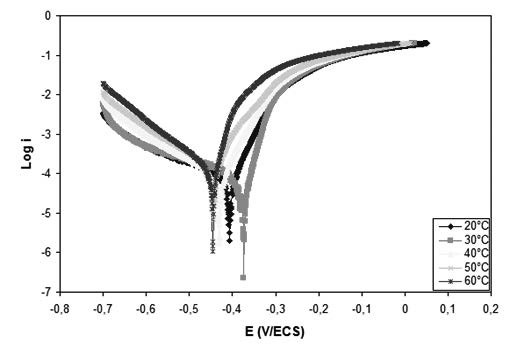
The inhibitive effect of date extract on the corrosion of Carbon steel in 1M HCl solution has been investigated by weight loss measurement, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. The presence of this extract reduces remarkably the corrosion rate of mild steel in acidic solution. The inhibition efficiency was found to increase with increasing inhibitor concentrations due to the adsorption of the inhibitor molecules on the metal surface, this adsorption follows Langmuir’s adsorption isotherm. The inhibition efficiency was found to be 85.84% at 1.5V/V% in 1M HCl.
Temperature studies revealed a decrease in efficiency with rise in temperature.
References
- Li. X, Deng. S, Fu. H, Li. T, Electrochim. Acta, 54, 4089, (2009).
- Vračar. L.M, and Dražić. D.M, Corros. Sci, 44, 1669, (2002).
- Behpour. M, Ghoreishi. S.M, Soltani. N, Salavati-Niasari. M, Corros. Sci, 51, 1073, (2009).
- Tang. Y.M, Yang. X.Y, Yang. W.Z, Wan. R, Chen. Y.Z, and Yin. X.S, Corros. Sci, 52 ,1801, (2010).
- Pavithra. M.K, Venkatesha. T.V, Punith Kumar. M.K, and Tondan. H.C, Corros. Sci, 60, 104, (2012).
- Cang. H, Fei. Z.H, Shao. J.L, Shi. W.Y, and Xu. Q, Int. J. Electrochem. Sci, 8, 720, (2013).
- Chaieb. E, Bouyanzer. A, Hammouti. B, Berrabah. M, Acta Phys. Chim. Sin, 25, 1254, (2009).
- El Hosary. A.A, Saleh. R.M, and Shams El Din. A.M, Corros. Sci, 12, 897, (1972).
- Ekpe. U.J, Ebenso. E.E, Ibok. U.J, J. West African Assoc, 37, 13, (1994).
- Loto. C.A, Nig. Cor. J, 19, 20, (1998).
- Chetouani. A, and Hammouti. B, Bull. Electrochem, 19, 23,(2003).
- Orubite. K.O, and Oforka. N.C, Mater. Lett, 58, 1768, (2004).
- Sharma. S.K, Mudhoo. A, Jain. G, and Sharma. J, Green Chem. Lett. Rev, 3, 7, (2010).
- Sivaraju. M, and Kannan. K, Int. J. Chem. Tech. Res, 2, 1243, (2010).
- Chuanhan. L.R, and Gunasekaran. G, Corros. Sci, 49, 1143, (2007).
- Abdel-Gaber. A.M, Abd-El Nabey. B.A, Sidahmed. I.M, El-Zayady. A.M, and Saadawy. M, Corrosion, 62, 293, (2006).
- Eddy. N.O, Port. Electrochim. Acta, 27, 579, (2009).
- Quraishi. M.A, ngh. Si.A, Singh. V.K, Yadav. D.K, and Singh. A.S, Mater. Chem. Phys, 122, 114, (2010).
- Satapathy. A.K, Gunasekaran. G, Sahoo. S.C, Amit. K, and Rodrigues. R.V, Corros. Sci, 51, 2848, (2009).
- Lebrini.M, Robert. F, Lecante. A, and Roos. C, Corros. Sci, 53, 687, (2011).
- Deng. S.D, X. and Li. H, Corros. Sci, 55, 407, (2012).
- Cang. H, Shi. W.Y, Shao. J.L, and Xu. Q, Int. J. Electrochem. Sci, 7, 3726, (2012).
- Ashassi-Sorkhabi. H, Seifzadeh. D, Int. J. Electrochem. Sci, 1, 92, (2006).
- Chetouani. A, Hammouti. B, Benkaddourm. M, Pigm. Resin Technol, 33, 26, (2004).
- Znini. M, Majidi. L, Bouyanzer. A, Paolini. J, Desjobert. J.M, Costa. J, Hammouti. B, Arabian. J. Chemistry, 5, 467, (2012).
- Raja. P.B, Sethuraman. G, Materials Letters, 62, 2977, (2008).
- Kalaiselvi. P, Chellammal. S, Palanichamy. S, Subramanian. G, Materials Chemistry and Physics, 120, 643, (2010).
- Khadraoui. A, Khelifa. A, Hamitouche. H, Mehdaoui. R, Res. Chem. Intermed, 40, 961, (2014).
- Da Rocha. J.C, Gums. J A. C, D’ Elia. E, Corrosion Science, 52, 2341, (2010).
- EL-ETRE. A.Y, Corrosion Science, 40, 1845, (1998).
- J. C. da Rocha, J. A. C. Ponciano Gomes, E. D’Elia, A. P. Gil Cruz, L. M. C. Cabral, A. G. Torres, M. V. C. Monteiro, Int. J. Electrochem. Sci. 7, 11941 (2012).
- Khadraoui, A. Khelifa, H. Boutoumi, H. Hamitouche, R. Mehdaoui, B. Hammouti, S.S. Al-Deyab, Int. J. Electrochem. Sci. 9, 3334 (2014).
- E.E. Oguzie , C.K. Enenebeaku, C.O. Akalezi, S.C. Okoro, A.A. Ayuk, E.N. Ejike, J.Coll.Interf. Sci. 349, 283 (2010).
- Langmuir. I, J. Am. Chem. Soc, 39, 184, (1947).
- Frumkin. A.N.Z, Phys. Chem, 116, 466, (1925).
- de Boer. J.H, The Dynamical Character of Adsorption, second ed. Claredon Press, Oxford, UK, 1968.
- Masel. R.I, Principles of Adsorption and Reaction on Solid Surfaces. Willey, New York, 1996.
- Belkhaouda. M, Bammou. L, Salghi. R, Zarrouk. A, Ebenso. Eno. E, Zarrok. H, Hammouti. B, Int. J. Electrochem. Sci, 8, 10999, (2013).
- Lebrini.M, Robert. F, Roos. C, Corros.Sci, 5, 1698, (2010).
- M. Ramananda Singh, J. Mater. Environ. Sci, 4, 119, (2013).


