EFFECT OF CONCENTRATION AND MOLECULAR MASS OF PEO ON THE MICELLIZATION AND THERMODYNAMIC BEHAVIOUR OF CETYLTRIMETHYLAMMONIUM BROMIDE (CTAB) IN AQUEOUS PEOCTAB MIXED SYSTEM
- Thermodynamics,
- Micellizatin,
- Surface tension,
- Hydrodynamic radius,
- Light scattering
- PEO ...More
Copyright (c) 2017 Abdur Rauf, Musa Kaleem baloch, Abbas Khan, Zafarullah Khan, Salma Rauf

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
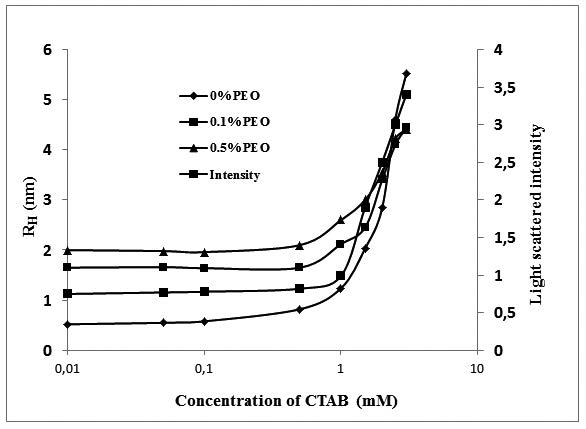
Polymer-surfactant mixed systems have attracted much attention due to their importance from the academic as well as applied point of view. Therefore, to evaluate the thermodynamic properties of polymer-surfactant self-assembled system, the critical micelle concentration (CMC) of CTAB and CTAB/PEO mixture was determined at different CTAB/PEO ratios and various molecular masses of PEO by employing surface tensiometric, conductometric, and light scattering techniques. The CMC of CTAB was increased as the concentration and molecular mass of the polymer increased. Using surface tensiometry, the thermodynamics of adsorption, surface excess concentration and the minimum area occupied by surfactant molecules for different surfactant-polymer mixtures were also evaluated. CMC, area per molecule, free energy of micellization and degree of counter ion binding were also determined. It was found that most of these parameters increased as the concentration of polymer increased, whereas the surface excess concentration was found to decrease by increasing the polymer ration in the mixed system. The hydrodynamic radius (Rh) of the micelle particles calculated from dynamic light scattering technique showed a similar trend. Most of the results from different techniques support each others.
References
- D. E. Goddard, In Interactions of Surfactants with Polymers and Proteins. Goddard. D.E.; Ananthapadmanabhan K.P.CRC Press: Boca Raton. FL, (1993).
- M. Goswami, J. M. Borreguero, P. A. Pincus, B. G. Sumpter, Macromol¬ecules, 48, 9050–9059 (2015).
- D. Li, N. J. Wagner, J. Am. Chem. Soc., 135, 17547–17555 (2013).
- U. Ashraf, O. A. Chat, M. Maswal, S. Jabeen, A. A. Dar, RSC Adv., 5, 83608-83618, (2015).
- M. K. Baloch, A. Bano, G. Hameed, J. Chem. Soc. Pak., 24, 77-86, (2002).
- M. Siddiq, C. Wu, M. Nawaz, M. K. Baloch, K. Mehmood, J. Chem. Soc. Pak., 23, 200-204, (2001).
- D. Langevin, Adv. Colloid Interface Sci., 89, 467-484, (2001).
- J. Fundin, W. Brown, Macromolecules, 27, 5024-5031, (1994).
- R. Roe, J. Polymer. Eng. Sci., 25, 1103-1109, (2004).
- Y. Bayrak, Turk. J. Chem., 27, 487-492, (2003).
- P. R. Desai, N. J. Jain, R. K. Sharma, P. Bahadur, Colloids Surf. A, Physicochem. Eng. Asp., 178, 57-69, (2001).
- M. S. Khan, Z. Ali, Chin. J. Polym. Sci., 23, 29-35, (2005).
- N. V. Sastry, H. Hoffmann, Colloids Surf. Physicochem. Eng. Asp., 250, 247-261, (2004).
- T. P. Lodge, Macromol. Chem. Phys., 204, 265-273, (2003).
- E. D. Goddard, J. Colloids Interf. Sci., 256, 228-240, (2002).
- C. L. Mesa, J. Colloids Interf. Sci., 286, 148-157, (2005).
- D. G. F. Taylor, R. K. Thomas, J. Penfold, Adv. Colloids Interf. Sci., 132, 69-110, (2007).
- J. Skerjanc, K. Kogej, G. Vesnaver, J. Phys. Chem., 92, 6382-6385, (1988).
- P. M. Macdonald, D. Staring, Y. Yue, Langmuir, 9, 381-384, (1993).
- X. Li, Z. Lin, J. Cai, L. E. Scriven, H. T. Davis, J. Phys. Chem., 99, 10865- 10878, (1995).
- R. Zana, In Structure-Performance Relationships in Surfactants, 2nd ed.; Esumi, K., Ueno, M., Eds.; Marcel Dekker: New York, (2003), p. 547.
- M. Benrraou, B. Bales, R., Zana, J. Colloids Interf. Sci., 267, 519-523, (2003).
- A. D. Aprano, C. L. Mesa, L. Persi, Langmuir, 13, 5876-5880, (1997).
- J. Mata, T. Joshi, D. Varade, G. Ghosh, P. Bahadur, Colloids Surf. Physicochem. Eng. Asp., 247, 1-7, (2004).
- A. Khan, G. Fatima M. Usman, W. Harrison M. Siddiq J. Chem. Soc. Pak., 31, 731-737, (2009).
- M. K. Baloch, A. Farzana A. Rauf, G. Fatima, G. Hameed, J. Appl. Polym. Sci., 116, 2133-2142, (2010).
- M. Usman, M. A. Cheema, A. Khan, Z. H. Farooqi, V. Mosquera, M. Siddiq, J. Chil. Chem. Soc., 58, 1842-1845, (2013).
- M. J. Rosen, Surfactants and interfacial phenomenon, New York, (1978).
- O. Kosaka, P. Sehgal, H. Doe, Food Hydrocolloid., 22, 144-149, (2008).
- M. Polverari, T.G. M. Van de Ven, J. Phys. Chem., 100, 13687-13695, (1996).
- H. Gharibi, N. Takisawa, P. Brown, M. A. Thomson, D. M. Painter, D. M. Bloor, D. G. Hall, E. Wyn-Jones, J. Chem. Soc. Faraday Trans., 87, 707- 710, E. (1991).
- N. Takisawa, D. G. Hall, E. W. Jones, P. Brown, J. Chem. Soc. Faraday Trans., 84, 3059-3070, (1988).
- H. Gharibi, Ph.D Thesis, “Electrochemical and Kinetic Studies in Surfactant Solution”, University of Salford, (1991).
- J. M. Del Rio, C. Pombo, G. Prieto, V. Mosquera, F. Sarmiento, J. Colloids Interf. Sci., 172, 137-141, (1995).
- W. Zhang, L. Ganzuo, M. U. Jianhai, Q. Shen, L. Zheng, H. Liang, W. Chi, Chin. Sci. Bull., 45, 1854-1857, (2000).


