PRELIMINARY RESULTS OF THE REACTION OF CYCLOTRIMERIZATION OF PHENYLACETYLENE [2+2+2] CATALYZED BY [(Cp*)Co(Indene)] COMPLEX
- cobalt complex,
- cyclotrimerization,
- [2 2 2] cycloadditions
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
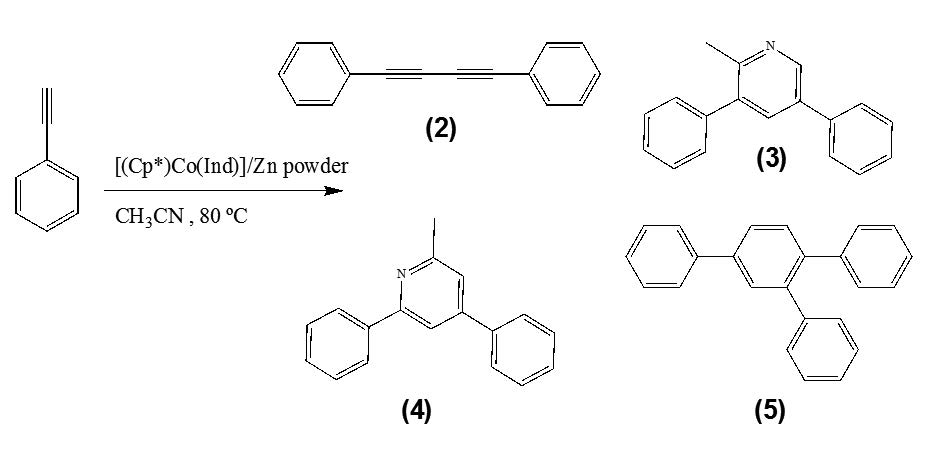
This work describes the catalytic study of [(Cp*)Co(Ind)] (with Cp*= pentamethylcyclopentadienyl, Ind= Indenyl, (C9H7)) complex in cyclotrimerization of phenylacetylene. From the cylcotrimerization reaction was possible to obtain products such as substituted pyridines 2-methyl-3,5-diphenylpyridine (3), 2-methyl- 4,6-diphenylpyridine (4) and the compound 1,2,4-triphenylbenzene (5) using acetonitrile as solvent. On the other hand, using toluene as solvent under the same working conditions, the product of reaction was 1,3,5-triphenylbenzene (1). Furthermore, by varying the working conditions, the reaction is 90% selective towards the formation of pyridines.
In addition, has been appreciated the formation of another product 1,4-diphenilbuta-1,3-diyne (2), which was isolated and characterized by means NMR and GC-Mass spectrometry.
References
- H. Bönnemman, Angew. Chem. Int. Ed. 24, 248 (1985).
- M. Lautens,W. Klute, W. Tam, Chem. Rev. 96, 49 (1996).
- V. Gevorgyan, U. Radhakrishnan, A. Takeda, M. Rubina, M. Rubin, Y. Yamamoto, J. Org. Chem. 66, 2835 (2001).
- Y. Yamamoto, Curr. Org. Chem. 9, 503 (2005).
- S. Kotha, E. Brahmachar, K. Lahiri, Eur. J. Org. Chem. 4741, (2005).
- P.R. Chopade, J. Louie, J. Adv. Synth. Catal. 348, 2307 (2006).
- V. Gandon, C. Aubert, M. Malacria, Chem. Commun. 2209 (2006).
- N. Agenet, V. Gandon, K. P. C. Vollhardt, M. Malacria, C. Aubert, J. Am. Chem. Soc. 129, 8860 (2007).
- Ph. Röse, C. C. Magraner Garcia, F. Pünner, K. Harms, G. Hilt, J. Org. Chem., 80, 7311 (2015).
- A. A. More, C. V. Ramana, J. Org. Chem., 81, 3400 (2016).
- K. P. C. Vollhardt, Angew. Chem., Int. Ed. Engl. 23, 539 (1984).
- N.E. Schore, Chem. Rev. 88, 1081 (1988).
- B.M. Trost, Science 254, 1471 (1991).
- N. Weding, M. Hapke, Chem. Soc.Rev. 40, 4525 (2011).
- G. Domíniguez, G.Peŕez-Castells, J. Chem. Soc. Rev. 40, 3430 (2011).
- M.S.B Wills, R.L. Danheiser. J. Am. Chem. Soc 120, 9378 (1998).
- Y. Sato, T. Tamura, M. Mori. Angew. Chem. Int. Ed. Engl. 43, 2436 (2004).
- A.G. Ardizzoia, S. Brenna, S. Cenini, G. LaMonica, N. Masciocchi, A. Maspero, J. Mol. Catal. A. Chemical. 204-205, 333 (2004).
- J. Varela, C. Saá. J. Organomet. Chem. 694, 143 (2009).
- G. Hilt, W. Hess, T. Vogler, C. Hengst, J. Organomet. Chem. 690, 5170 (2005).
- A. Stockis, R. Hoffmann, J. Am. Chem. Soc. 102, 2952 (1980).
- C. Bianchini, K.G. Caulton, C. Chardon, M.-L. Doublet, O. Eisenstein, S.A. Jackson, T.J. Johnson, A. Meli, M. Peruzzini, W.E. Streib, A. Vacca, F. Vizza,. Organometallics 13, 2010 (1994).
- J.M. O’Connor, K.D. Bunker, A.L. Rheingold, L. Zakharov, J. Am. Chem. Soc. 127, 4180 (2005).
- J.H. Hardesty, J.B. Koerner, T.A. Albright, G.-Y. Lee, J. Am. Chem. Soc. 121, 6055 (1999).
- H. Bönnemman. Angew. Chem. Int. Ed. 17, 505 (1978).
- M.S. Sigman, A.W. Fatland, B.E. Eaton. J. Am. Chem. Soc. 120, 5130 (1998).
- A.W. Fatland, B.E. Eaton, Org. Lett., 2, 3131 (2000).
- T. Tsuda, H. Maehara, Macromolecules 29, 4544 (1996).
- C. L. L. C. W.L.F. Armarego, Purification of Laboratory Chemicals, Fifth ed., (2003).
- Morales V. Study of the catalytic behavior of mononuclear organometallic complexes of Rh, Ni and Co in the dehydrogenative silylation of olefins (written in Spanish Estudio del comportamiento catalítico de complejos organometálicos mononucleares de Rh, Ni y Co en Sililación deshidrogenativa de olefinas), Ph.D. thesis. Pontificia Universidad Católica de Chile; (2010). https://www.researchgate.net/publication/321587889


