A THEORETICAL QUANTUM STUDY OF THE ELECTRONIC PROPERTIES OF MENTOXY DICHLORO PHOSPHOROUS (C10H19OPCl2)
- Phosphorous compound,
- DFT,
- HF,
- HOMO-LUMO
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
A theoretical quantum study of the organophosphorus compound with formula C10H19OPCl2 (MEPCL2) was carried out. The results of the calculations show excellent agreement between experimental and computed frequencies evaluated at the B3LYP/6-311++G(d,p) level of theory. A study of the electronic properties, such as excitation energies and wavelengths were performed employing the time-dependent DFT (TD-DFT) method. Global a chemical reactivity of MEPCL2 was analyzed through global reactivity descriptors, while its local reactivity was analyzed by mean maps of the electrostatic potential. Also, the orbital energies values suggest that a charge transfer is occurring within the molecule.
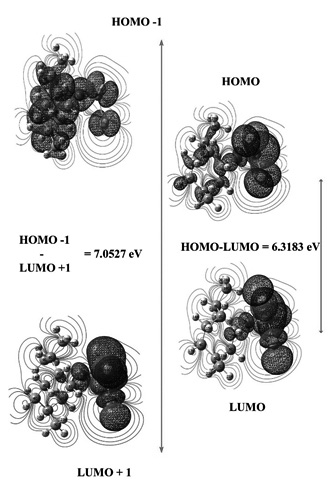
References
- Sengupta, S. K., Pandey, O. P., Rao, G. P., Vishen, P.: Metal Based Drugs. 8, 293 (2002).
- Suarez, P., Dullius, J.E. L., Einloft, S., De Souza, R., Dupont, R.: J. Inorg. Chim. Acta. 255, 207–209 (1997).
- Boon, A., Levisky, J. L., Pflug, L., Wilkes, J. S.: J. Inorg. Chem. 51, 480– 483 (1986).
- Yanes, E. G., Gratz, S. R., Baldwin, M. J., Robinson, S.E., Stalcup, A. M.: Anal. Chem. 73, 3838 (2001).
- Racke, K.D.: Degradation of organophosphorus insecticides in environmental matrices. in: Chambers, J.E., Levi, P.E. (eds.), Organophosphates: Chemistry, Fate, and Effects. Academic Press, San Diego. 47–73 (1992).
- Quin, L. D.: A Guide to Organophosphorus Chemistry; John Wiley & Sons, (2000).
- Mendoza-Huizar, L.H., J. Mex. Chem. Soc, 5(4) 416 (2014).
- Gaussian 03, Revision C.02, Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, Jr., J. A.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; and Pople, J. A.; Gaussian, Inc., Wallingford CT, 2004.
- Karabacak, M., Kurt, M.: Spectrochim. Acta A. 71, 876–883 (2008).
- Jamróz, M.H.: Vibrational Energy Distribution Analysis, VEDA 4, Warsaw, (2004).
- Kemp, W.: Organic Spectroscopy, Mac Millan Press Ltd., London, (1991).
- Shishkov, I.F., Sadova, N.I., Novikov, V.P., Vilkov, L.V., Strukt. Khim, Zh.: 25 (1984) 98–102.
- Sinha, L., Karabacak, M., Narayan, V., Cinar, M., Prasad, O.: Spectrochim. Acta 109A, 298–307 (2013).
- Socrates, G.: Infrared and Raman Characteristic Group Frequencies – Tables and Charts. third ed., Wiley, New York, (2001).
- Karabacak, M., Karagoz, D., Kurt, M.: J. Mol. Struct., 892, 25–31 (2008).
- Rani, A.U., Sundaraganesan, N., Kurt, M., Cinar, M., Karabacak, M.: Spectrochim. Acta A 75, 1523–1529 (2010).
- Balachandran, V., Lakshmi, A., Janaki, A.: Spectrochimica Acta A 81, 1–7 (2011).
- Bevan Ott, J., Boerio-Goates, J.: Calculations from Statistical Thermodaynamics, Academic Press, (2000).
- Martínez-Araya, J.I., Salgado-Morán, G., Glossman-Mitnik, D. J. Chem., 2013, 1 (2013).
- Krishnakumar, V., John Xavier, R.: Indian J. Pure Appl. Phys., 41, 597 (2003).
- Lashgari, A., Ghamami, S., Salgado-Morán, G., Ranirez-Tagle, R., Lorena Gerli – Candia, Journal of the Chilean Chemical Society, 61(1), 2821-2827 (2016).
- Lashgari, A., Ghammamy, S., Ramirez-Tagle, R., Salgado-Moran, G., Journal of Structural Chemistry, 56(8), 1505-1513, (2015).
- Ghamami, S., Shahbazkhany, S., Lashgari, A., International Journal of Pure and Applied Chemistry, 9(1-2), 57-62 (2014).
- Ghamami, S., Lashgari, A., Open Access Library Journal, 1: e422 (2014).
- Lashgari, A., Ghammamy, S., Shahsavari, M., Asian Journal of Research in Chemistry, 7(7), 677-680 (2014).


