- Croton elegans,
- alkaloid,
- secondary metabolite,
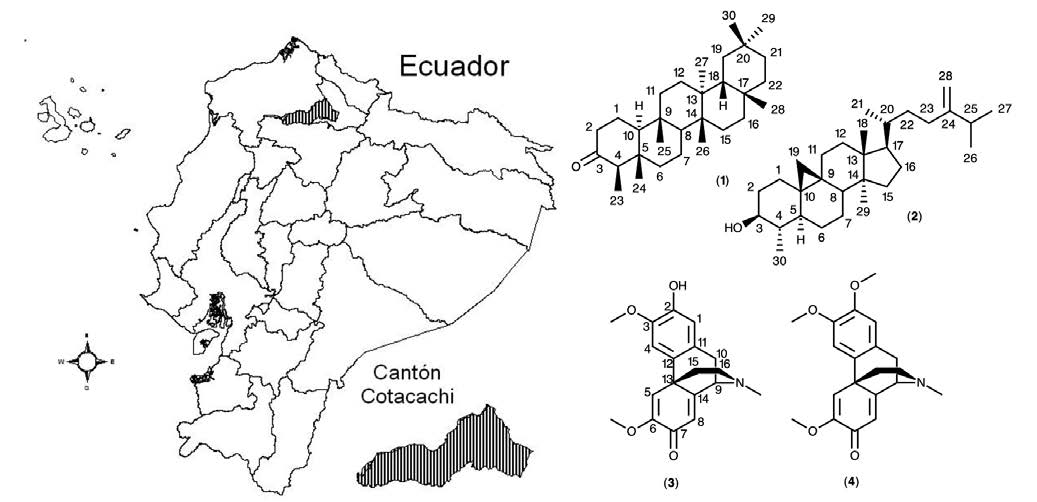
- Ecuador,
- NMR
- mosquera,
- friedelin,
- cycloeucalenol,
- ( )-pallidine,
- ( )-O-methylpallidine ...More
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
The species Croton elegans Kunth, belonging to the family Euphorbiaceae and known with the common name “mosquera”, is a native medicinal plant of Ecuador. Due to its medical applications, we are interested in secondary metabolite composition. After extraction with hexane and preparation of the alkaloid extract, preparative chromatographic fractionation in both normal and direct phase was performed. 1H NMR, 13C NMR, HSQC and HMBC experiments, together with polarimetric measurements, were conducted in order to characterize the purified compunds. Two triterpene metabolites (friedelin and cycloeucalenol) and two morphinan alkaloids ((+)-pallidine and (+)-O-methylpallidine) were obtained in a pure form. This work represents the first report on the phytochemistry of C. elegans and probably the second publication on the natural (+)-pallidine alkaloid.
References
- http://www.biodiversitya-z.org/content/megadiverse-countries.
- O. Malagón, J. Ramírez, J.M. Andrade, V. Morocho, C. Armijos, G. Gilardoni, Nat. Prod. Comm. 11, 297 (2016).
- P.M. Jørgensen, S. León-Yánez, Catalogue of the Vascular Plants of Ecuador, Missouri Botanical Garden Press, St. Louis, 1999.
- S. León-Yánez, R. Valencia, N. Pitman, L. Endara, C. Ulloa Ulloa, H. Navarrete (eds.) Libro rojo de las plantas endémicas del Ecuador, 2a edición. Publicaciones del Herbario QCA, Pontificia Universidad Católica del Ecuador, Quito, 2011.
- A. Salatino, M.L. Faria Salatino, G. Negri, J. Braz. Chem. Soc. 18, 11 (2007).
- H. Wagner, S. Bladt, Plant Drug Analysis - A Thin Layer Chromatography Atlas, Springer, Berlin, Heidelberg, 1996.
- E.J. Corey, J.J. Ursprung, J. Am. Chem. Soc. 78, 5041 (1956).
- Z. Wang, T. Yeats, H. Han, R. Jetter, J. Biol. Chem. 285, 29703 (2010).
- Y. Ding, C. Liang, J. Kim, Y.M. Lee, J.H. Hyun, H.K. Kang, J.A. Kim, B.S. Min, Y.H. Kim, Bioorg. Med. Chem. Letters 20, 1528 (2010).
- S. Martucciello, M.L. Balestrieri, F. Felice, C. Estevam, A.E. Goulart Sant’Ana, C. Pizza, S. Piacente, Chem. Biol. Inter. 183, 450 (2010).
- P. Antonisamy, V. Duraipandiyan, S. Ignacimuthu, J. Pharm. Pharmacol. 63, 1070 (2011).
- N. Ouédraogo, R.W. Sawadogo, A. Tibiri, C. Bayet, M. Lompo, A.E. Hay, J. Koudou, M.G. Dijoux, I.P. Guissou, Asian Pac. J. Trop. Med. , 46 (2012).
- E.A. Adewusi, P. Steenkamp, G. Fouche, V. Steenkamp, Nat. Prod. Comm. 8, 1213 (2013).
- H.F. Pi, P. Zhang, H.L. Ruan, Y.H. Zhang, H.D. Sun, J.Z. Wu, J. Asian Nat. Prod. Res. 11, 779 (2009).
- Y.H. Li, Y.F. Yang, K. Li, L.L. Jin, N.Y. Yang, D.Y. Kong, Chem. Pharm. Bull. 57, 401 (2009).
- T. Rukachaisirikul, A. Saekee, C. Tharibun, S. Watkuolham, A. Suksamrarn, Arch. Pharm. Res. 30, 1389 (2007).
- D. Menezes de Oliveira, W. da Nova Mussel, L.P. Duarte, G.D. Silva, H.A. Duarte, E. C. de Lima Gomes , L. Guimarães, S.A. Vieira Filho , Quim. Nova 35, 1916 (2012).
- T. Kikuchi, S. Kadota, K. Tsubono, Chem. Pharm. Bull. 34, 2479 (1986).
- K.L. Stuart, Chem. Rev. 71, 47 (1971).
- J. Liu, H. Wu, F. Zheng, W. Liu, F. Feng, N. Xie, J. Sep. Sci. 37, 2513 (2014).
- N. Ahmat Abdul Hamid, J. Latip, I.M. Said, L.B. Din, Malaysian J. Sci. 24, 33 (2005).
- J.P. Bartley, L.T. Baker, C.F. Carvalho, Phytochemistry 36, 1327 (1994).
- Z. Zhang, H.N. El Sohly, M.R. Jacob, D.S. Pasco, L.A. Walker, A.M. Clark, J. Nat. Prod. 65, 856 (2002).
- V. Vecchietti, C. Casagrande, G. Ferrari, B. Danieli, G. Palmisano, J. Chem. Soc. Perkin Trans. 1, 578 (1981).
- K.L. Stuart, C. Chambers, D. Byfield, J. Chem. Soc. (C), 1681 (1969).
- W.J. Eisenreich, G. Höfner, F. Bracher, Nat. Prod. Res. 17, 437 (2003).
- T. Kametani, M. Ihara, T. Honda, Chem Comm., 1301 (1969).
- X. Cheng, D. Wang, L. Jiang, D. Yang, Chem. Biodiv. 5, 1335 (2008).
- S.R. Hemingway, J.D. Phillipson, R. Verpoorte, J. Nat. Prod. 44, 67 (1981).
- M. Shamma, P. Chinnasami, S.F. Hussain, F. Khan, Phytochemistry 15, 1802 (1976).
- B. Franck, G. Dunkelinann, H.J. Lubs, Angew. Chem. 79, 1066 (1967).
- M. Bittner, M. Silva, P. Aqueveque, J. Kufer, J. Jakupovic, R. Murillo, Bol. Soc. Chil. Quim. 42, 223 (1997).
- M. Bittner, J. Alarcón, P. Aqueveque, J. Becerra, V. Hernández, M. Hoeneisen, M. Silva, Bol. Soc. Chil. Quim. 46, 419 (2001).


