A COAGULATION-FLOCCULATION PROCESS COMBINED WITH ADSORPTION USING ACTIVATED CARBON OBTAINED FROM SLUDGE FOR DYE REMOVAL FROM TANNERY WASTEWATER
- adsorption,
- coagulation-flocculation,
- dyes,
- leather industry,
- wet-end process
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Coagulation-flocculation (CF) process studies on aqueous solutions of Acid Black 210 dye (AB-210), were performed to investigate efficiency at different pH, coagulant and polyelectrolyte doses, and sedimentation times. In the adsorption studies of the dye removal, the initial pH and isotherms studies were performed using an activated carbon prepared by microwave-assisted pyrolysis of Tannery sludge. This adsorbent material was characterized by isotherms of N2 adsorption-desorption and density functional theory. Finally, the efficiency of the combined process CF followed by adsorption, was shown using a synthetic effluent identical to that produced in the wet-end process of the leather industry in industrial equipment. Effluent was collected from each treatment bath and analyzed for its physical properties and by ion chromatography.
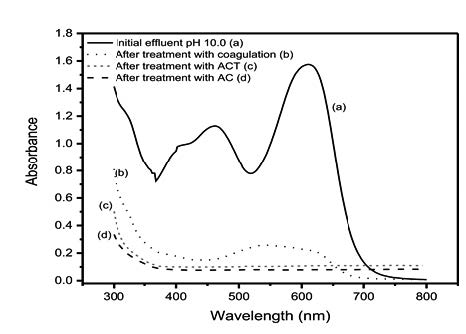
The best conditions found in CF processes were: pH 10.0, sedimentation time of 60 minutes, coagulant dose of 71 mg L-1 (Al2(SO4)3) and dose of flocculant (polyelectrolyte FX AS1) of 0.090 mg L-1. In the adsorption processes, the equilibrium data were best fit to the Liu isotherm model, presenting the maximum sorption capacity (Qmax) for AB-210 of 974.7 mg g-1 at 303.15K at pH 2.0. Using the synthetic effluent, the combined CF followed by adsorption of AB-210 dye demonstrated a removal of 85.2%, indicating that the combined process is efficient for real wastewater treatment.
References
- M. Gutterres, B. Mella, B. Chromium in Tannery Wastewater. Heavy Metals in Water: Presence, Removal and Safety. 1st ed. Cambridge: Royal Society of Chemistry, 2014; pp. 314-338.
- J. Piccin, C.S.Gomes, L.A Feris, M. Gutterres. Chem. Eng. J. 183, 30 (2012).
- Y.F., Feng, H. Zhou, G.H. Liu, J. Qiao, J.H. Wang, H.Y. Lu, L.Z. Yang, Y.H. Wu. Bioresour. Technol. 125, 138, (2012).
- X.Y. He, K.B. Male, P.N. Nesterenko, D. Brabazon, B. Paull, J.B.T. Luong. ACS Appl. Mater. Interfaces 5, 8796(2013).
- B.H. Tan, T.T. Teng, A.K.M. Omar. Water Res. 34, 597 (2000).
- J. Bratby, Coagulation and Flocculation in Water and Wastewater Treatment. Second ed., IWA Publishing, 2006.
- C. Liang, S. Sun, F. Li, Y. Ong, T. Chung. J. Membrane Sci., 469, 306 (2014).
- M. Rasool, B. Tavakoli, N. Chaibakhsh, A. Pendashteh, A. Ecol. Eng. 90, 431 (2016).
- I. Michael, A. Panagi, L.A. Loannou, Z. Frontistis, D. Fatta-Kassinos. Water Res. 60, 28 (2014).
- C. Amor, E. Torres-Socías, J. Peres, M. Maldonado, I. Oller, S. Malato, M. Lucas. J. Hazard. Mater. 286, 261 (2015).
- G. Han, C. Liang, T. Chung, M. Weber, C. Staudt, C. Maletzko, C. Water Res. 91, 361 (2016).
- S. Papic, N. Koprivanac, A.L. Bozic, A. Metes. Dyes pigm. 62 (3), 291(2004).
- F. Furlan, L. Melo da Silva, A. Morgado, A. Ulson de Souza. Resour. Conserv. Recy, 54, 283 (2010).
- C. Jung, N. Phal, J. Oh, K. Hoon, M. Jang, Y. Yoon. J. Hazard. Mater. 300, 808 (2015).
- V. Oloibiri, I. Ufomba, M. Chys, W. Audenaert, K. Demeestere, S. Van Hulle. Waste Manage. 43, 335 (2015).
- C. Saucier, M.A. Adebayo, E.C. Lima, R. Cataluna, P.S. Thue, L.D.T. Prola, M.J. Puchana-Rosero, F.M. Machado, F.A. Pavan, G.L. Dotto, J. Hazard. Mater. 289, 18 (2015).
- M.J. Puchana-Rosero, M.A. Adebayo, E.C. Lima, F.M. Machado, P.S. Thue, J.C.P. Vaghetti, C.S. Umpierres, M. Gutterres, Colloid Surf. A, 504, 105 (2016).
- M. C. Ribas, M. A. Adebayo, L. D.T. Prola, E. C. Lima, R. Cataluña, L. A. Feris, M.J. Puchana-Rosero, F. M. Machado, F. A. Pavan, T. Calvete, Chem. Eng. J. 248, 315 (2014).
- M.A. Adebayo, L.D.T Prola, E.C. Lima, M.J. Puchana-Rosero, R. Cataluña, C. Saucier, C.S. Umpierres, J.C.P. Vaghetti, L.G. da Silva, R. Ruggiero, J. Hazard. Mater. 268, 43 (2014).
- L.D.T. Prola, E. Acayanka, E.C. Lima, C.S. Umpierres, J.C.P. Vaghetti, W.O. Santos, S. Laminsi, P.T. Njifon, Ind. Crop. Prod., 46, 328 (2013).
- N.F. Cardoso, E.C. Lima, B. Royer, M.V. Bach, G.L. Dotto, L.A.A. Pinto, T. Calvete, J. Hazard. Mater. 241-242, 146 (2012).
- L.D. T. Prola, F.M. Machado, C.P. Bergmann, F.E. de Souza, C.R. Gally, E.C. Lima, M.A. Adebayo, S.L.P. Dias, T. Calvete, J. Environ. Manage. 130, 166 (2013).
- J. Rouquerol, P. Llewellyn, F. Rouquerol. Stud. Surf. Sci. Catal. 160, 49 (2007).
- M. Thommes, K. Kaneko, A. Neimark, J. Olivier, F. Rodríguez-Reinoso, J. Rouquerol, K. Sing. Pure Appl. Chem. DOI 10.1515/pac-2014 (2015).
- G. Gor, M. Thommes, K. Cychosz, A. Neimark. Carbon, 50, 1583 (2012).
- J. Landers, G. Yu, A. Neimark. Colloids and Surfaces A: Physicochem. Eng. Aspects. 437, 3 (2013).
- W.S. Alencar, E.C. Lima, B. Royer, B.D. dos Santos, T. Calvete, E.A. da Silva, C.N. Alves. Sep. Sci. Technol. 47,513 (2012).
- E.C. Lima, M.A. Adebayo, F.M. Machado. Kinetic and equilibrium models of adsorption. In: Bergmann CP, Machado FM, editors. Carbon nanomaterials as adsorbents for environmental and biological applications. Springer; 2015, pp.33–69.
- C. Saucier, M.A. Adebayo, E.C. Lima, L.D.T. Prola, P.S. Thue, C.S. Umpierres, M.J. Puchana-Rosero, F.M. Machado. Clean: Air, Soil, Water, 43, 1389 (2015).
- A. Zahrim, C. Tizaoui, N.J. Hilal. J. Hazard. Mater., 182, 624 (2010).
- J.M. Duan, J. Gregory. Adv. Colloid Interface Sci. 100,475 (2003).
- M.B Silveira, F.A. Pavan, N.F. Gelos, E.C. Lima, S.L.P. Dias, Clean: Air, Soil, Water, 42, 939 (2014).
- T. Calvete, E.C. Lima, N.F. Cardoso, S.L.P. Dias, E.S. Ribeiro. Clean: Air, Soil, Water, 38, 521 (2010).
- D. dos Santos, M.A. Adebayo, F. Pinheiro, L.D.T. Prola, R. Cataluña, E.C. Lima, C. Saucier, C. Gally, F. Machado. Korean J. Chem. Eng. 31, 1470 (2014).
- A. Bazzo, M.A. Adebayo, S.L.P. Dias, E.C. Lima, J.C.P. Vaghetti, E.R. de Oliveira, A.J.B. Leite, F.A. Pavan, Avocado seed powder: characterization and its application for crystal violet dye removal from aqueous solutions, Desalination and Water Treatment, 57, 15873 (2016).
- M. Shirmardi, N. Alavi, E.C. Lima, A. Takdastan, A.H. Mahvi, A.A. Babaei, Removal of atrazine as an organic micro-pollutant from aqueous solutions: a comparative study, Process Safety and Environmental Protection, 103, 23 (2016).
- P.A.M. Mourao, P.J.M. Carrot, M.M.L. Ribeiro Carrot. Carbon. 44, 2422 (2006).
- P.D. Húmpola, H.S. Odetti, A.E. Fertitta, J.L. Vicente. J. Chil. Chem. Soc., 58, 1, 1541 (2013).
- S. Gaydardzhier, J. Karthikeyan, P. Ay. Environ. Technol. 27 (2) ,193– 199.


