ADSORPTION OF As(III) FROM AQUEOUS SOLUTION ONTO IRON IMPREGNATED USED TEA ACTIVATED CARBON: EQUILIBRIUM, KINETIC AND THERMODYNAMIC STUDY
- As(III),
- Used Tea,
- Iron Oxide,
- Anion exchange,
- Sorption
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
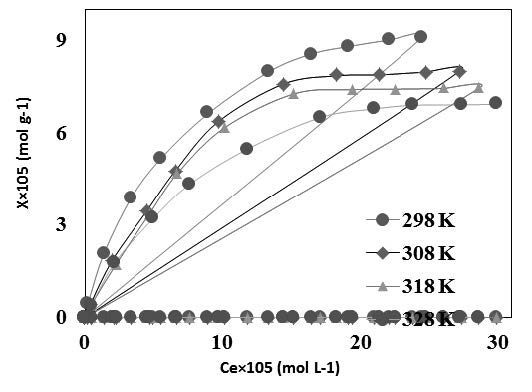
An efficient and cost effective adsorbent, activated carbon (UTAC) derived from used tea (UT) for the removal of As(III) from aqueous solutions was developed. To increase adsorption, UTAC was then impregnated with (magnetite) iron oxide particles (Fe-UTAC). The prepared adsorbents were characterized by XRD, SEM/EDX, FTIR and surface area analyzer. A comprehensive kinetic study of arsenite adsorption onto Fe-UTAC was conducted at 298-318 K and pH 8. The Richenberg model confirmed film diffusion to be the main rate limiting step. The removal of As(III) from aqueous solution onto Fe-UTAC was carried out as a function of temperature, concentration and pH. The sorption capacity (mol g-1) of Fe-UTAC was observed to increase with increase in arsenite concentration while a decrease in the As(III) uptake was observed by increasing the temperature of the system. The sorption capacity of Fe-UTAC was almost three and six times larger than that of UTAC and UT respectively. The effect of pH on the arsenite adsorption was significant in the pH range 7–8 which may be correlated with the stability of Fe-UTAC as no release of iron from Fe-UTAC was observed. The mean free energy calculated from DR mechanism confirmed adsorption to be chemisorption and followed ligand exchange mechanism. The thermodynamic parameters confirmed adsorption to be exothermic, spontaneous and favorable.
References
- Choi J, Yang JS, Park YT, Kim JO, Kim KJ, Shim YS, Kwon HH, Khan MA, Park JW, UM JG, Jeon BH. Comparison of As, Ni, Zn, Cd and Pb removals using treatment agents. Environ. Technol. 33, 445-454 (2012).
- Ghomri F, Lahsini A, Laajeb A, Addaou A. The removal of heavy metal ions (copper, zinc, nickel and cobalt) by natural bentonite. Larhyss J. 12, 37-54 (2013).
- Meher AK, Das S, Rayalu S, Bansiwal A. Enhanced arsenic removal from drinking water by iron-enriched aluminosilicate adsorbent prepared from fly ash. Desalin. Water Treat. 57, 20944-20956 (2016).
- Abid M, Niazi NK, Bibi I, Murtaza G, Kunhikrishnan A, Seshadri B, Shahid M, Ali S, Bolan NS, Ok YS, Abid M, Ali F. Arsenic (V) biosorption by charred orange peel in aqueous environments. Int J. Phytoremediat. 18, 442-449 (2016).
- De Oliveira LK, Melo CA, Goveia D, Lobo FA, Hernandez, MAA, Fraceto LF, Rosa AH. Adsorption/desorption of arsenic by tropical peat: influence of organic matter, iron and aluminium. Environ. Technol. 36, 149-59 (2015).
- Lunge S, Singh S, Sinha A. Magnetic iron oxide (Fe3O4) nano particles from tea waste for arsenic removal. J. Magn. Magn. Mater. 356 21–31 (2014).
- Ansari R, Sadegh M. Application of activated carbon for removal of arsenic ions from aqueous solutions. E-J Chem. 4, 103-108 (2007).
- Hossain I, Anjum N, Tasnim T. Removal of arsenic from contaminated water utilizing tea waste. Int. J. Environ. Sci. Technol. 13, 843–848 (2016).
- Polowczyk I, Bastrzyk A, Ulatowska J, Szczałba E, Koźlecki T, Sadowski Z. Influence of pH on arsenic (III) removal by fly ash. Separ. Sci. Technol. (2016). 10.1080/01496395.2016.1163610.
- Podder MS, Majumder CB. Sequestering of As(III) and As(V) from wastewater using a novel neem leaves/MnFe2O4 composite biosorbent. Inter. J. Phytoremediat. 18, 1237-1257 (2016).
- Salameh Y, Al-Muhtaseb AH, Mousa H, Walker GM, Ahmad MNM. Characterization of adsorption of aqueous arsenite and arsenate onto charred dolomite in microcolumn systems. Environ. Technol. 35, 3029- 3040 (2014).
- Hamayun M, Mahmood T, Naeem A, Mustafa S, Waseem M. Equilibrium and kinetics studies of arsenate adsorption by FePO4. Chemosphere 99, 207–215 (2014).
- Beduk F. Superparamagnetic nanomaterial Fe3O4-TiO2 for the removal of As(V) and As(III) from aqueous solutions. Environ. Technol. 37, 1790- 1801 (2016).
- Sanchez J, Toledo L, Bernabe LR, Rivera N , Munoz E. Water-soluble cationic cellulose coupled to a ultrafiltration membrane for the removal of arsenic and chromium. J. Chil. Chem. Soc. 58, 1986-1990 (2013).
- Dong L, Liu W, Jiang R, Wang Z. Study on the adsorption mechanism of activated carbon removing low concentrations of heavy metals. Desalin. Water Treat. 57, 7812-7822 (2016).
- Shakoor MB, Niazi NK, Bibi I, Murtaza G, Kunhikrishnan A, Seshadri B, Shahid M, Ali S. Bolan NS, Ok YS, Abid M, Ali F. Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit. Rev. Env. Sci. Technol. 46, 467-499 (2016).
- Prahas D, Kartika Y, Indraswati N, Ismadji S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 140, 32-42 (2008).
- Gomez-Serrano V, Cuerda-Correa EM, Fernandez-Gonzalez, MC, Franco MFA, Garcıa AM. Preparation of activated carbons from chestnut wood by phosphoric acid-chemical activation. Study of microporosity and fractal dimension. Mater. Lett. 59, 846-853 (2005).
- Bahri MA, Calvo L, Gilarranz MA, Rodriguez JJ. Activated carbon from grape seeds upon chemical activation with phosphoric acid: Application to the adsorption of diuron from water. Chem. Eng. J. 203, 348-356 (2012).
- Gupta VK, Pathania D, Sharma S. Adsorptive remediation of Cu(II) and Ni(II) by microwave assisted H3PO4 activated carbon. Arab. J. Chem. (2013) 10.1016/j.arabjc.2013.11.006.
- Dieme MM, Villot A, Gerente C, Andres Y, Diop SN, Diawara CK. Sustainable conversion of agriculture wastes into activated carbons energy balance and arsenic removal from water. Environ. technol. 10.1080/09593330.2016.1193225 (2016).
- Yu MR, Chang YY, Yang JK. Application of activated carbon impregnated with metal oxides to the treatment of multi contaminants. Environ. technol. 33, 1553-1559 (2012).
- Gundogdu A, Duran C, Senturk HB, Soylak M, Ozdes D, Serencam H, Imamoglu M. Adsorption of phenol from aqueous solution on a low-cost activated carbon produced from tea industry waste: Equilibrium, kinetic, and thermodynamic study. J. Chem. Eng. Data 57, 2733-2743 (2012).
- Mayakaduwa SS, Vithanage M, Karunarathna A, Mohan D, Ok YS. Interface interactions between insecticide carbofuran and tea waste biochars produced at different pyrolysis temperatures. Chem. Spec. Bioavailab. 28, 110-118 (2016).
- Akar E, Altinisik A, Seki Y. Using of activated carbon produced from spent tea leaves for the removal of malachite green from aqueous solution. Ecol. Eng. 52, 19-27 (2013).
- Panneerselvam P, Morad N, Tan KA. Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel(II) from aqueous solution. J. Hazard. Mater. 186, 160 – 168 (2011).
- Shamsuddin MS, Yusoff NRN, Sulaiman MA. Synthesis and characterization of activated carbon produced from kenaf core fiber using H3PO4 activation. Procedia Chem. 19, 558 – 565 (2016).
- Rajapaksha AU, Vithanage M, Zhang M, Ahmad M, Mohan D, Chang SX, Ok YS. Pyrolysis condition affected sulfamethazine sorption by tea waste biochars. Bioresour. Technol. 166, 303-308 (2014).
- Nethaji S, Sivasamy A, Mandal AB. Preparation and characterization of corn cob activated carbon coated with nano-sized magnetite particles for the removal of Cr(VI). Bioresour. Technol. 134, 94-100 (2013).
- Sulyman M, Namiesnik J, Gierak A. Utilization of new activated carbon derived from oak leaves for removal of crystal violet from aqueous solution. Pol. J. Environ. Stud. 23, 2223-2232 (2014).
- Soleimani M, Kaghazchi T. Agricultural waste conversion to activated carbon by chemical activation with phosphoric acid. Chem. Eng. Technol. 30, 649-654 (2007).
- Maiti A, Agarwal V, De S, Basu JK. Removal of As(V) using iron oxide impregnated carbon prepared from tamarind hull. J. Environ. Sci. Heal. A 45, 1207 – 1216 (2010).
- Mohan D, Sarswat A, Singh VK, Franco MA, Jr CUP. Development of magnetic activated carbon from almond shells for trinitrophenol removal from water. Chem. Eng. J. 172, 1111– 1125 (2011).
- Liu Z, Zhang FS, Sasai R. Arsenate removal from water using Fe3O4- loaded activated carbon prepared from waste biomass. Chem. Eng. J. 160, 57 – 62 (2010).
- Krejcova A, Tomas C. The determination of boron in tea and coffee by ICP–AES method. Food Chem. 82, 303–308 (2003).
- Malkoc E, Nuhoglu Y. Removal of Ni(II) ions from aqueous solutions using waste of tea factory: Adsorption on a fixed-bed column. J. Hazard. Mater. 135, 328-336 (2006).
- Marin MO, Gonzalez CF, Garcia AM, Serrano VG. Preparation of activated carbon from cherry stones by physical activation in air. Influence of the chemical carbonization with H2SO4. J. Anal. Appl. Pyrolysis 94, 131–137 (2012).
- Liou TH. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 158, 129–142 (2010).
- Karaagac O, Kockar H, Beyaz S. A Simple way to synthesize superparamagnetic iron oxide nanoparticles in air atmosphere: Iron ion concentration effect. IEEE Trans Magn. 46, 3978 – 3983 (2010).
- Gupta VK, Nayak A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Eng. J. 180, 81- 90 (2012).
- Podder MS, Majumder CB. Studies on the removal of As(III) and As(V) through their adsorption onto granular activated carbon/MnFe2O4 composite: isotherm studies and error analysis. Compos. Interf. 23, 327- 372 (2016).
- Mahmood T, Din SU, Naeem A, Mustafa S, Waseem M, Hamayun M. Adsorption of arsenate from aqueous solution on binary mixed oxide of iron and silicon. Chem. Eng. J. 192, 90 – 98 (2012).
- Ranjan D, Talat M, Hasan SH. Biosorption of arsenic from aqueous solution using agricultural residue rice polish. J. Hazard. Mater. 166, 1050 – 1059 (2009).
- Kundu S, Gupta AK. Adsorption characteristics of As(III) from aqueous solution on iron oxide coated cement (IOCC). J. Hazard. Mater. 142, 97-104 (2007).
- Maji SK, Pal A, Pal T, Adak A. Adsorption thermodynamics of arsenic on laterite soil. J. Surface Sci. Technol. 22, 161-176 (2007).
- Lin TF, Wu JK. Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics. Wat. Res. 35, 2049 – 2057 (2001).
- Liu X, Ao H, Xiong X, Xiao J, Liu J. Arsenic removal from water by iron-modified bamboo charcoal. Wat. Air Soil Pollut. 223, 1033-1044 (2012).
- Tawabini BS, Al-Khaldi SFA, Khaled MM, Atieh MA. Removal of arsenic from water by iron oxide nanoparticles impregnated on carbon nanotubes. J. Environ. Sci. Heal. A 46, 215-223 (2011).
- Kocabas ZO, Yurum Y. Kinetic Modeling of arsenic removal from water by ferric ion loaded Red Mud. Sep. Sci. Technol. 46, 2380-2390 (2011).
- Biswas BK, Junichi I, Katsutoshi I, Nath GK, Hiroyuki H, Keisuke O, Hidetaka K. Adsorptive removal of As(V) and As(III) from water by a Zr(IV)-loaded orange waste gel. J. Hazard. Mater. 154, 1066-1074 (2008).
- Ngantcha TA, Vaughan R, Reed BE. Modeling As(III) and As(V) removal by an iron oxide impregnated activated carbon in a binary adsorbate system. Sep. Sci. Technol. 46, 1419-1429 (2011).
- Tian Y, Wu M, Lin X, Huang P, Huang Y. Synthesis of magnetic wheat straw for arsenic adsorption. J. Hazard. Mater. 193, 10-16 (2011).
- Afzali D, Rouhani M, Fathirad F, Shamspur T, Mostafavi A. Nano iron oxide coated on sand as a new sorbent for removal of arsenic from drinking water. Desalin. Water Treat. 57, 13030-13037 (2016).
- Das B, Devi RR, Umlong IM, Borah K, Banerjee S, Talukdar AK. Arsenic (III) adsorption on iron acetate coated activated alumina: Thermodynamic, kinetics and equilibrium approach. J. Environ. Heal. Sci. Eng. 11, 1-10 (2013).


