SYNTHESIS, STRUCTURAL ELUCIDATION, CATALYTIC, ANTIBACTERIAL AND ANTIOXIDANT ACTIVITY OF THIOPHENE DERIVED MIXED LIGAND METAL COMPLEXES
- Mixed Ligand Complexes,
- Catalytic Oxidation,
- Antibacterial and DPPH radical Scavenging Activity
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
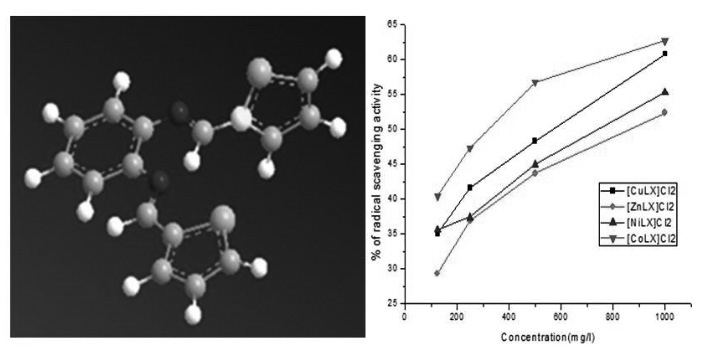
The hexa-coordinated mixed ligand Cu(II), Zn(II), Ni(II) and Co(II) complexes of the type [MLX]Cl2 where X=1,10-phenonthroline and ligand (L) have been synthesised from the condensation of thiophene-2-carboxaldehyde with o-phenylenediamine. They were characterised by elemental analysis, molar conductance, magnetic susceptibility, infrared, electronic absorption, proton magnetic resonance and mass spectral studies. An octahedral geometry has been proposed for all these complexes. The high molar conductance studies of the complexes show their electrolytic nature. The infrared spectra of the complexes show that the imine group of the Schiff base coordinate with the metal ion. The electronic absorption spectra of the complexes show the charge transfer, d-d transitions consistent with their proposed geometry. The proton magnetic resonance spectrum of the ligand shows the presence of CH=N group in the ligand. The mass spectral data also confirms the proposed structure of ligand and its complexes. The mixed ligand complexes possess good catalytic activity in the reaction of oxidation of alcohols using H2O2 as oxidant. The antibacterial activity of the mixed ligand complexes has been carried out by disc diffusion method. Copper (II) complexes exhibit potent antibacterial activity when compared to the standard drug streptomycin. The above complexes also exhibited DPPH radical scavenging activity.
References
- P. Krishnamoorthy, P. Sathyadevi, A.H. Cowley, R.R. Butorac and N. Dharmaraj, Eur. J. Med. Chem., 46, 3376, (2011).
- D. Lahiri, R. Majumdar, D. Mallick, T.K. Goswami, R.R. Dighe and A.R. Chakravarty, J. Inorg. Biochem., 105, 1086, (2011).
- C. Spinu, M. Pleniceanu and C. Tigae, Turk. J. Chem., 32, 487, (2008).
- B. Clarke, N. Clarke, D. Cunningham, T. Higgins, P. McArdle, M. Ni Cholchu and M. O’Gara, J.Organometallic Chem., 559, 55, (1998).
- D. Sathis Kumar and Ibrahim Sheriff A.K. ,Res. J. Chem. Sci., 6, 1, (2016).
- K. Singh, M.S. Barwa and P. Tyagi, Eur. J. Med. Chem., 42, 394, (2007).
- P.G. Cozzi, Chem. Soc. Rev., 33, 410, (2004).
- S. Chandra and J. Sangeetika, J. Indian Chem. Soc., 81, 203, (2004).
- F. Arjmand, F. Sayeed and M. Muddassir, J. Photochem.Photobiol. B, 103 (2011) 166.
- E. Canpolat and M. Kaya, J. Coord. Chem., 57, 1217, (2004).
- M. Yildiz, B. Dulger, S.Y. Koyuncu and B.M. Yapici, J. Indian Chem. Soc., 81 (2004) 7.
- Z. Lu and L. Yang, J. Inorg. Biochem., 95, 31, (2003).
- J.Z. Wu, H.Li, J.G. Zhang and H. Xu Ju, Inorg. Chem. Commun., 5, 71, (2002).
- L. Canali and D.C. Sherrington , Chem. Soc. Rev.,28, 85, (1999).
- A.A.Isse, A.Gennara and E.Viane, J. Electroanalytical Chem., 444, 241, (1998).
- R.Atkins, G.Brewer, E.Kokot, G.M.Mockler and E.Sinn, J.Inorg.Chem., 24, 127, (1985).
- J.P.Costes, G.Cros, M.H.Darbieu and J.P.Laurent, J.Inorg.Chim.Acta, 60, 111, (1982).
- E.Charles, S.George, E.Chester, N. Jyotirindra, J. American Chem. Soc., 12, 3233, (1955).
- A. Weissenberger, E.S. Proskauer, J.A. Riddick and E.E. Toops, Organic Solvents: Physical Properties and Methods of Purification, Techniques of Organic Chemistry, 3rd edn., Interscience, New York, 1955.
- A.I. Vogel, Practical Organic Chemistry, 4th edn., Longmann, 1978.
- R.J. Angellici, Synthesis and Techniques in Inorganic Chemistry, W.B. Saunders Company, 1969.
- A.I. Vogel, Text Book of Quantitative Chemical Analysis, 5th edn., Longmann, 1989.
- G. Qin, L. D. Fan, R. Zhang and S. K. Cao, Advanced Materials Research, 287-290, 1761, (2011).
- Corrado Crotti, Sergio Cenini, Fabio Ragaini, Francesca Porta and Stefano Tollari, J. Mol. Catal., 72, 283, (1992).
- Gopalakrishnan Karthikeyan and Ponnuchamy Pitchaimani, Transition Met. Chem., 28, 482, (2003).
- N.Raman, A.Kulandaisamy and K.Jeyasubramaniaan, Indian J. Chem., 41, 942, (2002).
- K. Sarkar, M. Nandi, M. Islam, M. Mubarak, A. Bhaumik, Appl. Catal., A 81, 352, (2009).
- K.C. Gupta, A.K. Sutar, C.C. Lin, Coord. Chem. Rev., 253, 1926, (2009).
- B. Bahramian, F.D. Ardejani, V. Mirkhani, K. Badii, Appl. Catal., A 97, 345, (2008).
- M. Salavati-Niasari, S.N. Mirsattari, J. Mol. Catal.A: Chem. 50, 268, (2007).
- M. Salavati-Niasari, M. Hassani-Kabutarkhani, F. Davar, Catal. Commun. 7, 955, (2006).
- M. Salavati-Niasari, P. Salemi, F. Davar, J. Mol. Catal.A: Chem. 215, 238, (2005).
- W.S. Kim, Y.-K. Choi, Appl. Catal., A 163, 252, (2003).
- C. Baleizo, H. Garcia, Chem. Rev., 106, 3987, (2006).
- T.C.O.M. Leod, D.F.C. Guedes, M.R. Lelo, R.A. Rocha, B.L. Caetano, K.J. Ciuffi, M.D Assis, J. Mol. Catal. A: Chem., 259, 319, (2006).
- C.Spinu, A.Kriza, Acta Chim. Slov.,47, 179, (2000).
- M.Hossain, S.K.Chattopadhyay, S.Ghosh,Polyhedron, 16, 1793, (1997).
- Mohammad Nasir Uddin, Didarul Alam Chowdhury, Md. Moniruzzman Rony and Md. Ershad Halim, Modern Chemistry, 2, 6, (2014).
- S. Chandra and R. Kumar, Transition Met. Chem., 29, 269, (2004).
- M.A.Neelakantan, S.S.Marriappan, J.Dharmaraja, T.Jeyakumar, K.Muthukumar, Spectra Chim. Acta Part A.,71, 628, (2008).
- B.J. Hathway, M. Duggan, A. Murphy, J. Mullane, C. Power, A. Walsh and B. Walsh, Coord. Chem. Rev., 36, 267, (1981).
- A.B.P. Lever, Inorganic Electronic Spectroscopy, 2nd edn, Elsevier, Amsterdam, 1984.
- F.A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 5th edn, Wiley, New York, 1988.
- D.Sathis Kumar, S.Rajiv Gandhi and Ibrahim sheriff, J.Chem. and Pharm. Research, 7(1), 416, (2015).
- D. Sathis Kumar and A. K. Ibrahim Sheriff, Der Pharma Chemica,7, 79, (2015).


