ESTIMATION OF NEWER ANTI-CANCER DRUG CLOFARABINE IN THEIR PHARMACEUTICAL DOSAGE FORM BY STABILITY INDICATING TLC METHOD
- Clofarabine,
- HPTLC,
- Validation,
- Analysis,
- Force Degradation
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
A sensitive, accurate, precise and reproducible high performance thin layer chromatographic method has been developed for the estimation of clofarabine in their pharmaceutical dosage form. The objective of this validation of an analytical procedure is to demonstrate that the drug Clofarabine is suitable for its intended purpose. The analytical method development recommends the quality, purity and specificity of the drug. Clofarabine injection form during the manufacturing process and hence the standard of drug may not vary, which produce the desirable therapeutic effect.TLC aluminum plates pre-coated with silica gel 60F254used as the stationary phase, while toluene: methanol (8:2, v/v) used as mobile phase. The Rf value was observe 0.34± 0.05 for clofarabine. The densitometric analysis was carried out in absorbance mode at 266 nm. The method was linear in the range of 50 – 1000 ng/spot for clofarabine. The method was validated as per ICH guideline. The limit of detection and limit of quantitation were found to be 17.60ng/spot and 53.35 ng/spot, respectively for clofarabine.The proposed method was successfully applied to the estimation of clofarabinein the pharmaceutical dosage form. Clofarabine was subjected to acid and alkali hydrolysis, chemical oxidation, wet hydrolysis, dry heat degradation and sun light degradation. The degradants peaks were well resolved from the pure drug peak with significant difference in their Rf values. Stressed samples were assayed using proposed TLC method.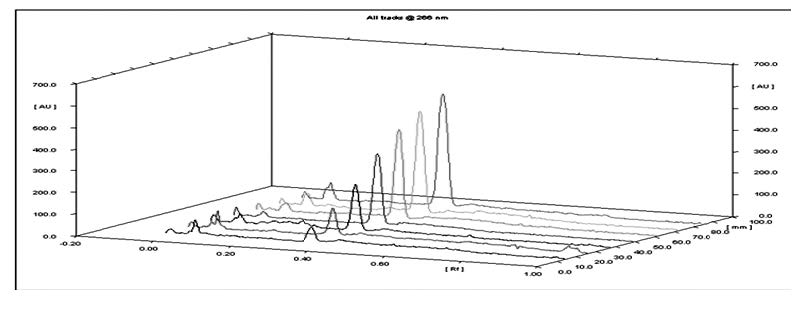
References
- Chemical and Physical Properties of Clofarabine, December 2004,http:// www.chemspider.com/Chemical-Structure.106472.html.
- Chemical property of Clofarabine, http://www.drugbank.ca/drugs/ DB00631.
- Shethi P. D., In HPTLC–High Performance Thin Layer Chromatography; 1st Edn; C.B.S Publishers, New Delhi, 1996, pp 1-68.
- Bailey L. C.., In Chromatography in the science and practice of pharmacy (Remington); 14th Edn; Mark Publishing Company, Pennsylvania, 1995, pp534-538.
- Jaenchen D. E., In Thin Layer (Planer) Chromatography in Handbook of Instrumental techniques for AnalyticalChemistry;1st Edn; Prentice Hall, New Jersey1997, pp 221-235.
- Renger B., J. Assoc. Official Agri. Chemist.76 (1993) 7-13.
- Stahl E., In Thin Layer Chromatography: A Laboratory Handbook; 2ndEdn; Springer International, Berlin, 1965, pp 1-30.
- ICH Guidelines Q2(R1), Validation of Analytical Procedures: Text and Methodology, Geneva, Switzerland, November2005.
- ICH–Guidelines Q1A (R2), Stability Testing of New Drug Substances and Products, International Conference on Harmonization, November Geneva, Switzerland (2003).
- Bakshi M, Singh S,. J. Pharm. and Biomed Anal. 28 (2002) 1011-1040.
- Reynolds D. W., Facchine K. L., Mullaney J. F., Alsante J. F., Hatajik T. D. and Motto M. G., Pharmaceutical. Technology. 26(2) (2002) 48–54.
- Baertschi S. W., Alsante K. M., Pharmaceutical Technolog. 27(2) (2003) 60-72.
- Karen M., Akemi A., Roland B., Janice E. Todd D., Adv Drug Del. Rev. 59(1) (2007) 29–37.
- Waterman K. C., Adami R., Int. J. Pharma. 293(1- 2) (2005) 101-125.


