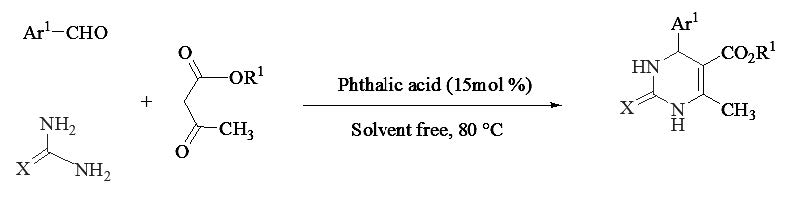
PHTHALIC ACID: A GREEN, BIODEGRADABLE AND ENVIRONMENTALLY BENIGN NATURE DIFUNCTIONAL BRØNSTED ACID CATALYST FOR THE ONE-POT SYNTHESIS OF 3, 4-DIHYDROPYRIMIDIN-2- (1H)-ONE DERIVATIVES AND SUBSTITUTED DIHYDRO-2-OXYPYRROLES
- Phthalic acid,
- 3,
- 4-Dihydropyrimidinone,
- Substituted dihydro-2-oxypyrrole,
- Biodegradable green catalyst
- Multi-component reactions,
- Green chemistry ...More
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Phthalic acid as a green, biodegradable economical and environmentally benign nature catalyst for the one-pot three-component Biginelli synthesis of 3,4-dihydropyrimidin-2-(1H)-one derivatives via β-keto esters, aldehyde derivatives and urea/thiourea under thermal and solvent-free conditions and one-pot four-component domino condensation of substituted dihydro-2-oxypyrrole by reaction of dialkyl acetylenedicarboxylate, formaldehyde and amines (aromatic and aliphatic) under ambient temperature with short reaction times and excellent yields is reported. The most benefits this procedure are such as green, biodegradable, inexpensive and non-toxic catalyst, eco-friendly, high catalytic activity, efficient, easily separation with no column chromatographic separation, simple operational procedures, one-pot, excellent yields, environmentally benign nature.
References
- M.D. Nikam, P. Mahajan, A.V. Chate, S. Dabhade, C. Gill, J. Chil. Chem. Soc. 60, 2847, (2015).
- M. Nasr-esfahani, M. Montazerohori, M. Aghel-mirrezaee, H.Kashi, J. Chil. Chem. Soc. 2014, 59, 2311, (2014).
- P.S. Anikt, N. Jaiprakash, N.B. Sangshetti, A.P. Dharbale, P.S. Sarkate wakte, D.B. Shinde, J. Chil. Chem. Soc. 2013, 58, 2200, (2013).
- Z. Madanifar, M.T. Maghsoodlou, M. Kangani, N. Hazeri, Res. Chem. Intermed. DOI: 10.1007/s11164-015-1993-6 (2015).
- P. Biginelli Gazz Chim Ital. 23, 360, (1893).
- S. Wisen, J. Androsavich, C.G. Evans, L. Chang, J.E. Gestwicki , Bioorg. Med. Chem. Lett.18, 60, (2008).
- O. Prakash, R. Kumar, V. Parkash, Eur. J. Med. Chem. 43, 435, (2008).
- A.D. Borthwick, A.J. Crame, P.F. Ertl, A.M. Exall, T.M. Haley, G.J. Hart, A.M. Mason, M.K. Pennell, O.M.P. Singh, G.G. Weingarten, J.M. Weingarten, J. Med. Chem. 45, 1, (2002).
- R. Shiraki, A. Sumino, K. Tadano, S. Ogawa, Tetrahedron Lett. 36, 5551, (1995).
- Y. Chen, D.X. Zeng, N. Xie, Y.Z. Dang, J. Org. Chem. 70, 5001, (2005).
- B.B. Snider, B.J. Neubert, J. Org. Chem. 69, 8952, (2004).
- I.A. Khodja, R. Boulcina, A. Debache, J. Chem. Pharm. Res. 6,1040, (2014).
- Y. Zhang, B. Wang, X. Zhang, J. Huang, C. Liu, Molecules. 20, 3811, (2015).
- S.B. Singh, M.A. Goetz, E.T. Jones, G.F. Billes, R.A. Giacobbe, L. Herranz, S. Stevens Miles, D.L. Williams, J. Org. Chem. 60, 7040, (1995).
- S. Chitra, K. Pandiarajan, TetrahdronLett. 50, 2222, (2009).
- A. Kamal, T. Krishnaji, M.A. Azhar, J.Catal. Commun. 8, 1929, (2007).
- J. Lai, M. Sharma, S. Gupta, P. Parashar, P. Sahu, D.D. Agarwal, J. Mol. Catal. A. Chem. 352, 31, (2012).
- A. Kumar, R.A. Maurya, Tetrahedron Lett. 48, 4569, (2007).
- M. Litvic, I. Vecenaj, Z.M. Ladisic, M. Lovric, V. Vinkovic, M. Filipan- Litvic,Tetrahedron. 66, 3463, (2010).
- C.J. Liu, J.D. Wang, Molecules. 14, 763, (2009).
- A.T. Khan, A. Ghosh, M. Musawwer Khan, Tetrahedron Lett. 53, 2622, (2012).
- Q. Zhu, H. Jiang, J. Li, S. Liu, C. Xia, M. Zhang, J. Comb. Chem. 11, 685, (2009).
- S. S. Sajadikhah, N. Hazeri, J. Res. Chem. Intermed. 40, 737, (2014).
- S.S. Sajadikhah, N. Hazeri, M.T, Maghsoodlou, S.M. Habibi Khorasani, A. Beigbabaei, A. C. Willis., J. Iran. Chem. Soc. 10, 863, (2013).
- S.S. Sajadikhah, N. Hazeri, M.T. Maghsoodlou, J. Chem. Res. 37, 40, (2013).
- S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, S. Mohamadian-Souri, Res. Chem. Intermed. DOI: 10.1007/s11164-015-2178-z (2015).
- S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, Chin. Chem. Lett. 25, 58, (2014).
- S. Sajjadifar, M.A. Zolfi gol, A. Ghorbani-Choghamarani, A.R. Banaei, S. Karimian, S. Miri Scientia. Iranica. 21, 2005, (2014).
- S. Sajjadifar, S. Miri, Int. J. Chem. Tech. Res. CODEN (USA): IJCRGG. 6, 5433 (2014).
- F.M. Danikuu, E.N.K. Sowley, J. Med & Biomed. Sci. 3, 9, (2014).


