HIGH THROUGHPUT ULTRA PERFORMANCE LIQUID CHROMATOGRAPHY - MASS SPECTROMETRIC METHOD FOR DETERMINATION OF ADRENALINE AND CHARACTERIZATION OF ITS STRESS DEGRADATION PRODUCTS: A MECHANISTIC APPROACH
- Adrenaline,
- UPLC-MS method,
- pharmaceutical analysis,
- forced degradation study
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
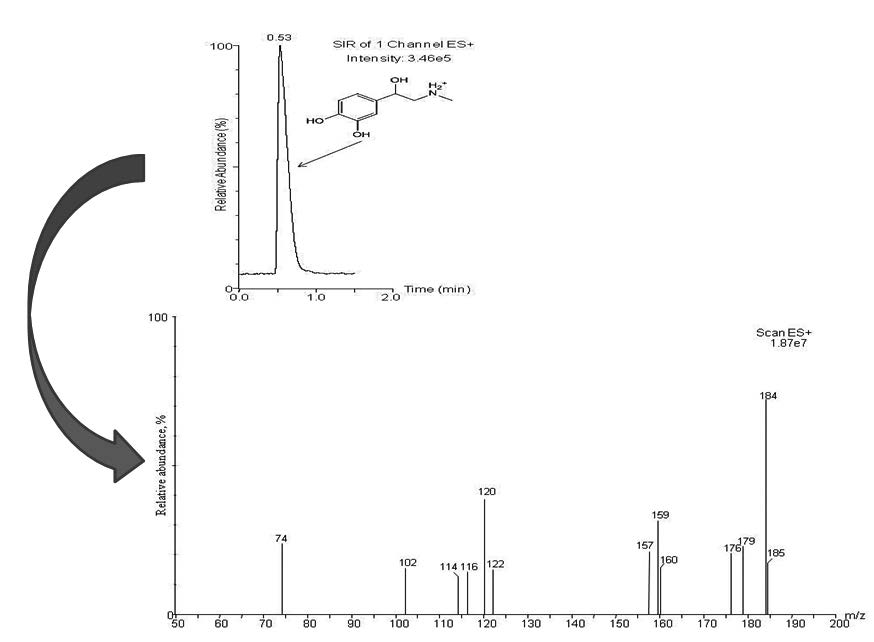
UPLC-MS analytical procedure has been established for the determination of adrenaline in marketed formulation and in the pure form. The detection of the adrenaline was executed considering positive electrospray ionization (ESI) and selected ion reaction (SIR) modes. Chromatographic separation was achieved using ACQUITY UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm particles size) using a binary mobile phase mixture of methanol and water (50:50). The mobile phase was set to flow at the rate of 0.3 ml min-1. The run time time for adrenaline was as short as 1.5 minutes. Linearity was found to be over the concentration range of 1μg ml-1 - 6μg ml-1. An excellent recovery of 100.18 % - 100.68 % was achieved using this procedure. The standard deviation ranged from 0.014 - 0.118 in intra-day studies and 0.016 - 0.096 in case of inter-day studies. The procedure’s quantitation limit was 0.76μg ml-1 and detection limit was 0.25 μg ml-1. The method was successfully applied to determine adrenaline in drug formulations. In order to get the information about the stability of adrenaline in various environments forced degradation studies were conducted and the characterization of the stressed samples were performed using mass spectrometry.
References
- K.H. Berecek, M.J. Brody. Am. J. Physiol. 242, H593,(1982).
- T. Yamashima. J. Takamine. J. med. Biog. 11, 95, (2003).
- Y. K. Kothari, K. Srinivasulu. Asian J Chem. 1, 42, (1989).
- S. A. H. Al-Ameri. Arab. J. Chem. (2011) Article in press.
- M. J. Hamzah, A. B. M. Mahood, S. A. Abid. J. karbala univ. 7, 9-14, (2009).
- M.R. H. Nezhad, J. Tashkhourian and J. Khodaveisi. J. Iran. Chem. Soc. 7, S83-S91, (2010).
- S. R El-Shabouri, S. A. Hussein, A. A. Abdel-Alim. J. – Assoc. Off. Anal. Chemists - Canary Database.71, 764, (1988).
- M. E. Al-Kommos, F. A. Mohamed, A. S. Khedr. Talanta. 37, 625, (1990).
- J. J. Berzas Nevado, J. M. Lemus Gallego, P. Buitrago Laguna. Anal. Chim. Acta. 300, 293, (1995).
- J. J. Berzas Nevado, J. M. Lemus Gallego, P. Buitrago Laguna. J. Pharm. Biomed. Anal.14, 571, (1996).
- A. Ruiz Medina, M. L. Fernandez de Cordova, and A. Molina Diaz. Sensitive Mikrochem. Acta. 134, 101, (2000).
- L. K. Abdulrahman, A.M. Al-Abachi, M.H. Al-Qaissy. Anal. Chim. Acta. 538, 331. (2005).
- H. J. Vieira, O. Fatibello-Filho. Eclet. Quim. 29, 79, (2004).
- A. K. Mishra, A. Mishra, P. Chattopadhyay. Arch. Appl. Sci. Res., 2, 251, (2010).
- J. Westermann, W. Hubl, N. Kaiser, L. Salewski. Clin. lab. 48, 61, (2002).
- Y. Guo, J. yang, X. Wu, A. Du. J. Fluorescence. 15,131, (2005).
- Å. Bertler, A. Carlsson, E.Rosengren. Acta Physiol. Scand. 44, 273, (1958).
- J. Yang, G. Zhang, X. Wu, F. Huang, C. Lin, X. Cao, L. Sun, Y. Ding. Anal. Chim. Acta. 363,105, (1988).
- B. J. Petteys, K. S. Graham, M.L. Parnás, C. Holt, E.L Frank. Clin. Chim. Acta. 413, 1459, (2012).
- C. Ji, J. Walton, Y. Su, M. Tella. Anal. Chim. Acta. 670, 84, (2010).
- M. R. Siddiqui, M. Z. Rafiquee, S. M. Wabaidur, Z. A. Alothman, M. S. Ali, H. A. Allohedan. Anal Sci. 31, 437 (2015).
- R. Bavandpour, H. Karimi-Maleh, M. Asif, V. K. Gupta, N. Atar, M. Abbasghorbani, J. Mol Liq. 213, 369 (2016).
- Z. Chen, Y. Hu, Q.Yang, C.Wan, Y.Tan, H. Ma. Sensors and Actuators B: 207, 277 ( 2015).
- M.Z.A. Rafiquee, M.R. Siddiqui, M. S. Ali, H. A. Al-Lohedan. Spectrochim Acta A.126, 21 (2014)..
- C. Yang, F.Chen, Z. Chang, Y. Sun, Z. Zhang. Spectrochim. Acta Part A 121, 5, 288 (2014).
- M. R.Siddiqui, S.M. Wabaidur, Z. A. Alothman, H.Rahman, Md. Sarfaraz Alam, Md. Sajid Ali. J. Chil. Chem. Soc., Mar 2014, vol.59, no.1, p.2303- 2307.
- ICH topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. C.J. Briscoe, M.R. Stiles, D.S. Hage, J. Pharm. Biomed. Anal., 44, 484, (2007).
- Abdullah S. Al-Ayed, Hamad A. Al-Lohedan, M.Z.A.Rafiquee, M. Sajid Ali, Zuheir A. Issa, Transition Met. Chem., 38, 173–181 (2013).


