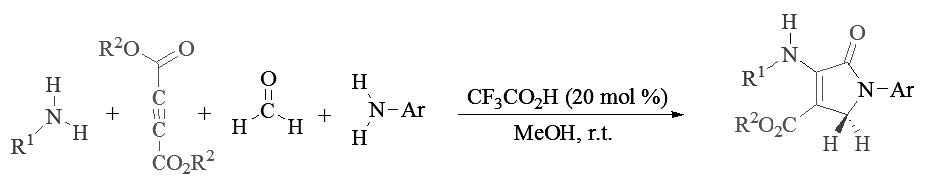
TRIFLUOROACETIC ACID CATALYZED ONE-POT FOUR-COMPONENT DOMINO REACTION FOR THE SYNTHESIS OF SUBSTITUTED DIHYDRO 2-OXYPYRROLES
- Trifluoroacetic acid,
- N-aryl/alkyl-3-aminodihydropyrrol-2-one-4-carboxylates,
- ambient temperature
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Trifluoroacetic acid was applied as an efficient catalyst for the one-pot four-component synthesis of N-aryl/alkyl-3-aminodihydropyrrol-2-one-4-carboxylates via the domino reaction of amines, formaldehyde and dialkyl acetylenedicarboxylates at ambient temperature in methanol. This methodology includes number of advantages such as: short reaction time, clean work-up, use of inexpensive catalyst, high yields and clean work-up. The work-up of this reaction involves only a filtration and a simple washing step with MeOH, and there is no need for column chromatography.
References
- T. Eicher, S. Hauptmann, Wiley-VCH, Weinheim, (2003).
- P.Z. Zhang, S.F. Zhou, T.R. Li, L. Jiang, Chin. Chem. Lett. 23, 1381 (2012).
- T.N. Akhaja, J.P. Raval, Design, Chin. Chem. Lett. 23, 446 (2012).
- W.J. Bai, S.K. Jakson, T.R.R. Pettus, Org. Lett. 14, 3862 (2012).
- M. Aginagalde, T. Bello, C. Masdeu, Y.Vara, A. Arrieta, F. Cossío J. Org. Chem. 75, 7435 (2010).
- H. Anaraki-Ardakani, M. Noei, A. Tabarzad, Chin. Chem. Lett. 23, 45 (2012).
- L. Ettlinger, E. Ga¨uemann, R. Hu¨tter, W. Keller-Schierlein, F. Kradolfer, L. Neipp, V. Prelog, H. Zähner, Helv. Chim. Acta 42, 563 (1959).
- H. Shiozawa, S. Takahashi, J. Antibiot. 47, 851 (1994).
- S.B. Singh, M.A. Goetz, E.T. Jones, G. F. Bills, R. A. Giacobbe, L.H. Siobhan Stevens- Miles, D. L. Williams, J. Org. Chem. 60, 7040 (1995).
- H. He, H.Y. Yang, R. Bigelis, E. H. Solum, M. Greenstein, Tetrahedron Lett. 43, 1633 (2002).
- A.J. Clark, C.P. Dell, J.M. McDonagh, J. Geden, P. Mawdsley, Org. Lett. 5, 2063 (2003).
- J. Chen, P.Q. Huang, Y. Queneau, J. Org. Chem. 74, 7457 (2009).
- A. Raghuraman, E. Ko, L.M. Perez, T. R. Ioerger, K. Burgess, J. Am. Chem. Soc. 133, 12350 (2011).
- T. Kawasuji, M. Fuji, T. Yoshinaga, A. Sato, T. Fujiwara, R. Kiyama, Bioorg. Med. Chem. 15, 5487 (2007).
- L. Zhang, Y. Tan, N.X. Wang, Q.Y. Wu, Z. Xi, G.F. Yang, Bioorg. Med. Chem. 18, 7948 (2010).
- Y. Mizushina, S. Kobayashi, K. Kuramochi, S.Nagata, F. Sugawara, K. Sakaguchi, Biochem.Biophys. Res. Commun. 273, 784 (2000).
- Q. Zhu, L. Gao, Z. Chen, S. Zheng, H. Shu, J. Liet, Eur. J. Med. Chem. 54, 232 (2012).
- B. Li, M.P.A. Lyle, G. Chen, J. Li, K. L. T. Hu, M. A. Alaoui-Jamali, J. Webster, Bioorg. Med. Chem. 15, 4601 (2007).
- A.S. Demir, F. Aydigan, I.M. Akhmedov, Tetrahedron: Asymmetry 13, 601 (2002).
- T.R.K. Reddy, C. L, X. Guo, X. Myrvang, H.K. P. M. Fischer, L. V. Dekker, J. Med. Chem. 54, 2080 (2011).
- Q. Zhu, H. Jiang, J. Li, S. Liu, C. Xia, M. Zhang, J. Comb. Chem. 11, 685 (2009).
- A.T. Khan, A. Ghosh, Md.M. Khan, Tetrahedron Lett. 53, 2622 (2012).
- H. Gao, J. Sun, C.G. Yan, Tetrahedron 69, 589 (2013).
- S. Rana, M. Brown, A. Dutta, A. Bhaumik, C. Mukhopadhyay, Tetrahedron Lett. 54, 1371 (2013).
- L. Lv, S. Zheng, X. Cai, Z. Chen, Q. Zhu, S. Liu, ACS Comb. Sci. 15, 183 (2013).
- R. Doostmohammadi, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi- Khorassani, Res. Chem. Intermed., 38, 4061 (2012).
- S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, S. M. Habibi-Khorassani, A.C. Willis, Chin. Chem. Lett. 23, 569 (2012).
- M.T. Maghsoodlou, S.M. Habibi-Khorasani, Z. Shahkarami, N. Maleki, M. Rostamizadeh, Chem. Lett. 21, 686 (2010).
- S.S. Sajadikhah, N. Hazeri, M.T. Maghsoodlou, S.M. Habibi-Khorassani, A. Beigbabaei, A.C. Willis, J. Iran. Chem. Soc. 10, 863 (2013).
- S.S. Sajadikhah, N. Hazeri, Res. Chem. Int. 40, 737(2014).
- S.S. Sajadikhah, N. Hazeri, M.T. Maghsoodlou, S.M. Habibi-Khorassani, J. Chin. Chem. Soc. 60, 1003 (2013).
- For a review, see: (a) T. Wu, H. Yu, C. Li, ARKIVOC ix (2004) 60– 65; (b) P.E. Peterson, C. Casey, E.V.P. Tao, A. Agtarap, G. Thompson, J. Am. Chem. Soc. 87 (1965) 5163–5169; (c) P.E. Peterson, R.J. Bopp, D.M. Chevli, E.L. Curran, D.E. Dillard, R.J. Kamat, J. Am. Chem. Soc. 89 (1967) 5902–5911.
- (a) For a review, see: K. Turnbull, D.M. Krein, Synthesis (1999) 391–392; (b) R. Venkateswarlu, C. Kamakshi, P.V. Subhash, S.G.A. Moinuddin, M.P. Gowri, R.S. Ward, A. Pelter, M.B. Hursthouse, S.J. Coles, M.E. Light, Tetrahedron 61 (2005) 8956–8961; (c) A.M. Martin-Castro, Chem. Rev. 104 (2004) 2939–3002; (d) R. Pathak, S. Madapa, S. Batra, Tetrahedron 63 (2007) 451-460.
- For a review, see: (a) R.D. Wakharkar, M.B. Sahasrabuddhe, H.B. Borate, M.K. Gurjar, Synthesis (2004) 1830–1834; (b) V.V.N.K.V.P. Raju, V. Ravindra, S.S. Kamath, V.T. Mathad, P.K. Dubey, P.P. Reddya, ARKIVOC xii (2009) 296–301; (c) A.K. Singh, R.E. Weaver, G.L. Powers, V.W. Rosso, C. Wei, D.A. Lust, A.S. Kotnis, F.T. Comezoglu, M. Liu, K.S. Bembenek, B.D. Phan, D.J. Vanyo, M.L. Davies, R. Mathew, V.A. Palaniswamy, W.-S. Li, K. Gadamsetti, C.J. Spagnuolo, W.J. Winter, Org. Proc. Res. Dev. 7 (2003) 25–27.
- For a review, see: (a) S.A. Bowden, J.N. Burke, F. Gray, S. McKown, J.D. Moseley, W.O. Moss, P.M. Murray, M.J. Welham, M.J. Young, Org. Proc. Res. Dev. 8 (2004) 33-44; (b) G.T. Bourne, D.J. Kuster, G.R. Marshall, Chem. Eur. J. 16 (2010) 8439–8445; (c) R.N. Misra, D.B. Rawlins, H.- Y. Xiao, W. Shan, I. Bursuker, K.A. Kellar, J.G. Mulheron, J.S. Sack, J.S. Tokarski, S.D. Kimball, K.R. Webster, Bioorg. Med. Chem. Lett. 13 (2003) 1133–1136; (d) K.G. Akamanchi, N.R. Varalakshmy, B.A. Chaudhari, Synlett (1997) 371–372; (e) M.N. Masuno, T.F. Molinski, Tetrahedron Lett. 42 (2001) 8263–8266; (f) J.P. Patel, A.-H. Li, H. Dong, V.L. Korlipara, M.J. Mulvihill, Tetrahedron Lett. 50 (2009) 5975–5977.
- For a review, see: (a) A.A. Zagulyaeva, M.S. Yusubov, V.V. Zhdankin, J. Org. Chem. 75 (2010) 2119–2122; (b) G.A. Olah, Q. Wang, N.J. Trivedi, G.K.S. Prakash, Synthesis (1991) 739–740; (c) R.A. Kjonaas, A.E. Clemons, J. Chem. Ed. 85 (2008) 827–828.
- For a review, see: (a) K. Li, L.N. Foresee, J.A. Tunge, J. Org. Chem. 70 (2005) 2881–2883; (b) T. Hashimoto, S. Kutubi, T. Izumi, A. Rahman, T. Kitamura, J. Organometal. Chem. 696 (2011) 99–105.
- For a review, see: (a) M.R. Mohammadizadeh, A. Hasaninejad, M. Bahramzadeh, Synth. Commun. 39 (2009) 3232–3242; (b) J. Salazar, S.E. Lo´ pez, O. Rebollo, J. Fluorine Chem. 124 (2003) 111–113; (c) J. Ohtaka, T. Sakamoto, Y. Kikugawa, Tetrahedron Lett. 50 (2009) 1681–1683; (d) S.E. Lo´ pez, Y. Pe´ rez, J. Restrepo, J. Salazar, J. Charris, J. Fluorine Chem. 128 (2007) 566–569; (e) F. Herna´ ndez-Luis, A. Herna´ ndez- Campos, R. Castillo, G. Navarrete-Va´zquez, O. Soria-Arteche, M. Herna´ ndez-Herna´ ndez, L. Ye´pez-Mulia, L. Eur, J. Med. Chem. 45 (2010) 3135–3141.
- For a review, see: (a) X.-G. Zhang, H.-X. Dai, M. Wasa, J.-Q. Yu, J. Am. Chem. Soc. 134 (2012) 11948–11951; (b) M. Eisenberg, D.D. Desmarteau, Inorg. Nucl. Chem. Lett. 6 (1970) 29–34; (c) A. Gregoric, M. Zupan, J. Org. Chem. 44 (1979) 4120–4122; (d) T.B. Patrick, K.K. Johli, D.H. White, W.S. Bertrand, R. Mokhtar, M.R. Kilboourn, M.J. Welch, Can. J. Chem. 64 (1986) 138–141; (e) Y. Tanabe, N. Matsuo, N. Ohno, J. Org. Chem. 53 (1988) 4582–4585; (f) N.V. Kondratenko, E.P. Vechirko, L.M. Yagupolskii, Synthesis (1980) 932; (g) F. Cottet, M. Schlosse, Eur. J. Org. Chem. (2002) 327–330.
- S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, 2013, DOI 10.1007/ s11164-013-1364-0.
- H.-J. Wang, L.-P. Mo, Z.-H. Zhang, ACS Comb. Sci. 13, 181 (2011).
- S. Rana, M. Brown, A. Bhaumik, C. Mukhopadhyay. Tetrahedron Lett. 54 (2013) 1371-1379.
- S.S. Sajadikhah, N. Hazeri, M.T. Maghsoodlou, S.M. Habibi-Khorassani, K. Khandan-Barani, J. Chem. Res., (2013) 40.
- S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, Chin. Chem. Lett., 25 (2014) 58.


